 |
de | fr | en print view![]()
3R-INFO-BULLETIN 34
January 2007
Authors

Yara Banz, carried out this project as part of her MD-PhD thesis together with PD Dr. Robert Rieben.
Robert Rieben’s research group has been investigating the potential of endothelial cell protectants for the last 10 years. Projects are carried out in close collaboration with clinical partners, mainly the Clinic of Cardiovascular Surgery at the Bern University Hospital.
The support for the present project from Prof. André Haeberli and Mrs. Trinh Cung from the Department of Clinical Research at the University of Bern, as well as from Prof. Nicolai Bovin and Dr. Elena Korchagina at the Russian Academy of Sciences in Moscow, is kindly acknowledged.
Current address:
Dr. Yara Banz
ybanz@bidmc.harvard.edu
present address:
Beth Israel Deaconess Medical Center
Harvard University 99 Brookline Avenue
02215 Boston, MA
USA
PD Dr. Robert Rieben
robert.rieben@dkf.unibe.ch
Dept. of Clinical Research
Cardiovascular Surgery
Inselspital
University of Bern
CH-3010 Bern
Switzerland
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation
Exploring natural anticoagulation by endothelial cells: A novel in vitro model
Compounds which protect vascular endothelial cells are highly desirable[*]. Until today the activity of such substances had to be tested in animal models because of complex interactions between endothelial cells, coagulation and innate immunity. In project no. 81-02 a novel in vitro approach was established by replacing flatbed cultures through cultivation of endothelial cells on microcarrier beads. By that the cell surface-to-blood volume ratio reach that of small arteries and veins and suffices to ensure ‘natural’ anticoagulation in whole blood. This allows the interaction between blood cells and derived natural factors and interactions with endothelial cells to be studied in vitro.
The endothelium’s natural anticoagulant properties
The resting endothelium effectively maintains an anti-inflammatory and anti-coagulant intravascular environment. This state is mainly upheld by the endothelial surface layer, the glycocalyx, which is composed of glycolipids, proteoglycans and associated glycosaminoglycans. The association of soluble factors from the blood with the glycocalyx secures help to maintain an anti-coagulatory state of the endothelium in vivo.
Preservation of anticoagulant properties in vitro
In conventional flatbed culture systems, work with whole, non-anticoagulated blood leads to coagulation as the endothelial surface per blood volume does not suffice to ensure that the endogenous anticoagulant state can be maintained. Accordingly, the interaction between blood cells and derived natural factors and interactions with endothelial cells cannot be studied in conventional cell culture systems. However, replacing flatbed cultures through cultivation of endothelial cells on microcarrier beads increases the surface-to-blood volume ratio twenty-fold to reach that of small arteries and veins (16 cm2 endothelial cell surface per ml of blood). This endothelial surface suffices to ensure ‘natural’ anticoagulation in whole blood, rendering the use of anticoagulants unnecessary.
Set-up of whole blood in vitro assay
The system is based on endothelial cells (as an example, porcine aortic endothelial cells, PAEC) which are previously expanded in vitro in conventional flatbed culture systems. Once a sufficient cell number is available, the cells are transferred to spinner flasks and mixed with collagen-coated Biosilon microcarrier beads (5 ml, equal to 800 cm2, Nalge Nunc International) and cultured until the cells form a confluent monolayer on the beads. Washed beads are then be incubated with non-anticoagulated, freshly withdrawn whole human blood at a bead-to-blood volume ratio of 1:4. Samples of the bead-blood mixture are taken at regular intervals during the experiment. The beads can be analyzed by immunofluorescence for various cell activation markers, cell integrity etc., while the plasma is used to analyze the coagulation/inflammatory status. In this system potential “endothelial cell protectants”, i.e. substances which preserve endothelial integrity and prevent activation of the endothelial cells, can be pre-incubated with the cells in the flask or co-incubated with the whole blood, and their effects assessed.
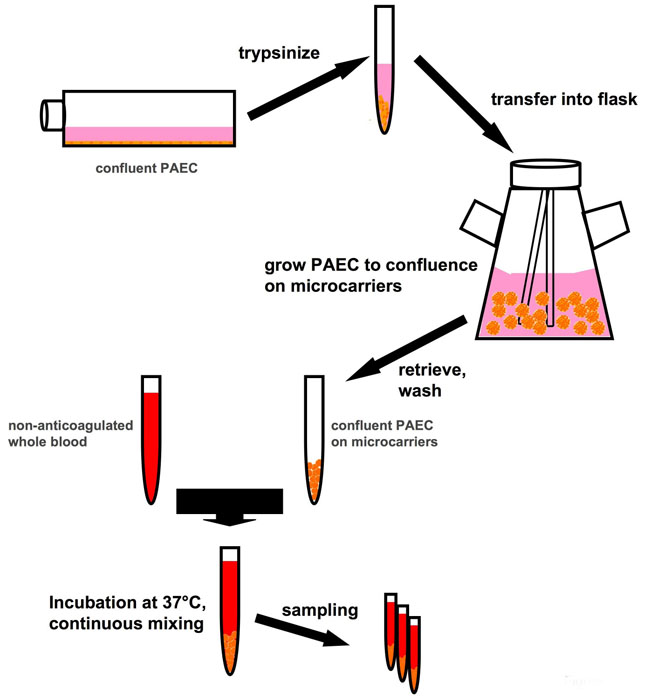
Fig. 1: Schematic representation of the test principle. Porcine aortic endothelial cells (PAEC, used here as an example of endothelial cells in general) are expanded in conventional flatbed cell culture and then grown to confluence on microcarriers. PAEC-coated microcarriers can then be incubated with whole, non-anticoagulated blood, exploiting the natural anticoagulant properties of the endothelium.
Search for “endothelial cell protectants”
Conventionally, (pre-)screening of substances with respect to their effects as endothelial protectants needed to be performed in vivo in animal models of e.g. ischemia/reperfusion injury, transplantation, or shock. All of them induce a high discomfort to the animals. The novel in vitro EC culture system allows for the screening of such substances without the use of animal models. Compounds identified may be candidates for later application in vivo.
Preventing activation of cascade systems
One strategy in tackling the problem of endothelial damage is to prevent activation of the cascade systems, mainly complement and coagulation. In collaboration with academic partners in the field of bioorganic chemistry our laboratory has been developing soluble complement/coagulation inhibitors and analyzing their potential role as "endothelial cell protectants". As an example low molecular weight dextran sulfate (DXS), a sulfated polysaccharide which inhibits complement activation in human serum1, prevents hyperacute rejection of pig lungs xenoperfused with human blood and significantly reduces the extent of tissue damage in a porcine model of acute myocardial infarction 2, 3. DXS was also tested in vitro in our EC-bead system 4 (see below). Several other, fully synthetic substances have been developed and tested for their anti-coagulatory and anti-complement effects using conventional in vitro systems. Further testing is now required to see which of these substances act as EC protectants and have the potential for clinical application. This information can now be acquired in the described in vitro system before choosing the most promising agents and reverting to in vivo animal models for confirmation.
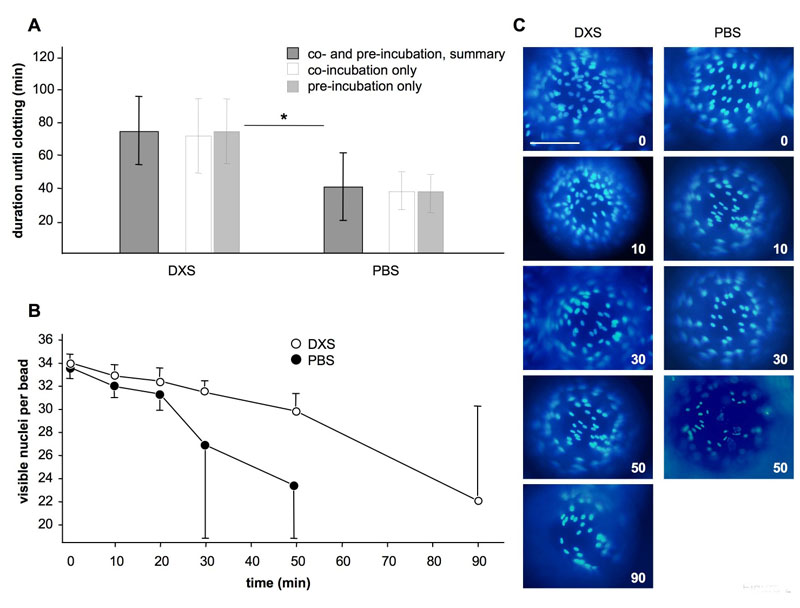
Fig. 2: Effect of the endothelial cell protectant DXS in the in vitro model with whole blood. (A) Duration of experiments in minutes until occurrence of clotting. Shown are averages and standard deviations for experiments with DXS and PBS controls. *P < 0.05 for DXS vs. PBS. (B) Amounts of nuclei per bead for DXS- and PBS experiments at different time-points during the experiments, as counted on the surface visible in panel C. (C) Representative images of individual beads with DAPI stained cell nuclei at baseline (0 min), after 10, 30, 50 and 90 min of incubation with human blood. Scale bar represents 100 μm.
Mimicking xenotransplantation in vitro
The addition of non-anticoagulated human blood to beads coated with porcine aortic endothelial cells was used to mimic a xenotransplantation setting in our in vitro model. Human blood contains naturally occurring antibodies which bind to galactosyl antigens on porcine cells and activate the complement cascade. The subsequent activation of the endothelial cells leads to a loss of their anticoagulant surface layer of heparan sulfate proteoglycans and therefore initiates the coagulation cascade. in vivo this is the hallmark of hyperacute rejection and a major reason for the failure of pig-to-human xenotransplantation. In our in vitro system a loss of the PAEC from the polystyrene beads, complement deposition on the beads and a loss of von Willebrand factor expression on the remaining PAEC on the beads were observed. In addition, clotting of the human blood occurred within a few minutes. Time until clotting can be used as an indicator of endothelial cell activation and damage. Addition of our 'prototype' endothelial cell protectant DXS significantly prolonged time until clotting as shown in figure 2A and reduced cell loss from the beads (Figure 2B/C).
3R benefit and limitations
The described in vitro model is currently suitable for the analysis of acute endothelial cell damage like antibody- and complement-mediated (hyper)acute vascular rejection or for studying certain aspects of ischemia/reperfusion injury. Most probably due to contact with air, blood clotting occurs within about 90 minutes even with the use of DXS as an endothelial cell protectant. Situations in which endothelial damage takes several hours or days to occur can therefore not be mimicked with the current model. In addition, flow conditions and shear stress which may play an important role in vivo are not present in the model. Also the phenomenon of vascular leakage, in which the endothelial cells stay intact but loose their barrier function due to intracellular gap formation, cannot be mimicked. Nevertheless, the possibility to do a first screening with substances aimed at protecting endothelial cells from ischemia/reperfusion injury or antibody- and complement-mediated attack, offered by our new in vitro model, has the potential to significantly reduce the number of animals derived from animal models. Furthermore, mechanistic studies can be performed much easier in vitro, which in addition reduces the use of animals in the respective areas of research.
Published updated version of this Bulletin 34/2007 (PDF)
References:
- Laumonier T, Walpen AJ, Maurus CF, Mohacsi PJ, Matozan KM, Korchagina EY, Bovin NV, Vanhove B, Seebach JD, Rieben R. Dextran sulfate acts as an endothelial cell protectant and inhibits human complement- and NK cell-mediated cytotoxicity against porcine cells. Transplantation. 2003;76:838-843.
- Fiorante P, Banz Y, Mohacsi PJ, Kappeler A, Wuillemin WA, Macchiarini P, Roos A, Daha MR, Schaffner T, Haeberli A, Mazmanian GM, Rieben R. Low molecular weight dextran sulfate prevents complement activation and delays hyperacute rejection in pig-to-human xenotransplantation models. Xenotransplantation. 2001;8:24-35.
- Banz Y, Hess OM, Robson SC, Mettler D, Meier P, Haeberli A, Csizmadia E, Korchagina EY, Bovin NV, Rieben R. Locally targeted cytoprotection with dextran sulfate attenuates experimental porcine myocardial ischaemia/reperfusion injury. Eur Heart J. 2005;26:2334-2343.
- Banz Y, Cung T, Korchagina EY, Bovin NV, Haeberli A, Rieben R. Endothelial cell protection and complement inhibition in xenotransplantation: a novel in vitro model using whole blood. Xenotransplantation. 2005;12:434-443.
| [*] | Endothelial cells in diseaseEndothelial cells form the inner lining of blood vessels, the endothelium, which is the interface between circulating blood and surrounding tissue. Endothelial cells are involved in control of blood pressure, coagulation, atherosclerosis, angiogenesis and inflammation. Endothelial activation, leading to dysfunction and damage, has been demonstrated in several different clinical situations:
In vivo, endothelial cell activation is generally linked to both the complement system (a biochemical cascade of the immune system) and the blood coagulation cascade. By activation-induced shedding of the endothelial surface layer of heparan sulfate proteoglycans, dendritic cells in the blood may undergo maturation and transform into efficient antigen presenting cells. Endothelial cell damage is therefore linked to activation of innate immune defense mechanisms as well as to blood clotting. So far, studying interactions between the endothelium on one hand and the coagulation system, the complement pathways, antibodies, and other players of the innate immune system on the other hand inevitably necessitated the use of in vivo models. This is because conventional ex vivo or in vitro systems can only operate with serum, plasma or anticoagulated whole blood. With the coagulation system and/or the critical interaction with blood cells “out of bounds”, the results derived from such in vitro experiments paint an incomplete picture. The challenge, therefore, was to establish an in vitro model for endothelial cell activation and damage, which allows the interactions of whole blood with endothelial cells to be investigated under conditions comparable to in vivo. |
| Last modified 2008/01/30 |