 |
de | fr | en print view![]()
Annual report 2002
1. Structure of the Foundation
The Foundation is a cooperative institution set up by the Parliamentary Group for Animal Experimentation Questions (public organ), Interpharma (Novartis Pharma Ltd, F. Hoffmann-La Roche Ltd, Serono Ltd) and the Foundation for Animal-Free Research (animal protection). It was entered in the commercial register on 18th August, 1987.
The funds provided to subsidise research stem from the Federal Veterinary Office and Interpharma.
2. Purpose of the Foundation
The purpose of the 3R Research Foundation Switzerland is to promote alternative research methods which avoid the use of animals through grants for research projects. The organisation supports first and foremost projects aimed at developing new methods or refining accepted methods (validation) which offer practical improvements vis-à-vis standard animal experimentation in line with the 3R motto Reduce, Refine, Replace.
A broad range of projects is sponsored on the condition that they are likely to reduce the number of animals used or the stress and/or pain suffered. Projects considered must be based on the Foundation's three principles and are mainly in the bio-medical multidisciplinary field.
3. Activities during 2002
In its sixteenth year of existence the Administrative Board met twice, in April and December, for a half-day meeting. Apart from the statutory business concerning the end of the business year 2001, the Board addressed the following issues.
Research funds for 2002 were allotted to 10 projects already underway. In addition, 4 new projects were approved, while 9 applications were rejected. The Board also took note of the final assessment by the Evaluation Committee of 4 projects which had been completed. In April administrative questions in connection with the submission of projects were clarified through a change in the guidelines. In December the Administrative Board received a new promise of funds from Interpharma amounting to Sfr. 2.4 million. Together with the corresponding contributions from the Federal Veterinary Office, this means that the continuation of the Foundation's activities is ensured.
The scientific adviser represented the Foundation at the 4th World Congress on Alternatives and Animal Use in the Life Sciences held in August 2002 in New Orleans. In November 2002 the 3R Research Foundation became a member of the European consensus platform for 3R alternatives (ECOPA) based in Brussels, which has been re-founded on the basis of new statutes. This means that it is now part of a European network along with Austria, Belgium, the Czech Republic, Denmark, Germany, Finland, Italy, the Netherlands, Norway, Poland, Spain, Sweden and the UK (14 countries in all). The aim of this association is to promote the international exchange of information about projects relevant to the 3R principle and to facilitate paneuropean efforts and coordination.
With the support of the scientific advisor, the Evaluation Committee held two meetings during the year, where in particular they assessed new applications and evaluated completed projects. The scientific adviser's tasks included publishing the 3R Info Bulletin (as a brochure and on the Foundation's Internet site at www.forschung3r.ch), writing brief scientific reports in English which present the projects receiving funding and keeping the Foundation's Internet site up-to-date. He was also busy working with Oekosophie GmbH to create a training model for 3R requirements via the Internet (module for further training of specialists who carry out animal experiments). Much time was also devoted to advising applicants and project leaders, calling for intermediate reports, evaluating draft projects and dealing with enquiries and explanations for the rejection of applications. In addition, the scientific adviser was involved in the preparatory work concerning the re-founding of ECOPA (drawing up statutes, preparing for the first General Meeting). Finally, he represented the Foundation at several meetings in Switzerland and abroad.
During the year 2 projects were completed (58/97 and 66/99). Together with those projects completed earlier (1-5/87, 6-15/88, 16/89, 17-20/90, 21-24/91, 25-42/92, 43-44/95, 45-51/96, 53-55/96, 57/97, 59-62/97 64/97, 65/98, 68/99 and 74/00) this brings the total of finished projects to 65 out of 84.
4. Personnel
In 2002 Dr. Raymond F. Miserez resigned as representative of the Federal Veterinary Office on the Administrative Board and the Evaluation Committee since he has taken on a new post at the Federal Office. The latter proposed Ursula Moser, B.Sc. to replace him in both functions, and this nomination was approved. At the end of their terms of office (December 2002) National Councillor Stephanie Baumann and Prof. Andreas Steiger decided not to stand for a further period on the Administrative Board and the Evaluation Committee respectively.
5. 3R-Info-Bulletin
So far the following 3R Information Bulletins have been published in English:
6. Financial business
During 2002 around Sfr. 502,000 was used for research grants. An additional Sfr. 80,000 represented an initial payment for setting up an Internet solution for 3R training. Project supervision and information accounted for around Sfr. 95,000 and administrative expenses totalled Sfr. 58,000. Total expenditure was consequently around Sfr. 735,000. The amount spent on research for current projects (Sfr. 472,000) was thus approximately Sfr. 154,000 below budget. This is mainly due to the fact that funding for two projects was Sfr. 130,000 less than expected. Furthermore, the 5% reserve was not paid out owing to the late submission of the final report. On the other hand, it was possible to allot a sum of Sfr. 30,000 to a new project. Operational expenditure for project supervision, information and administration totalled around Sfr. 153,000 and was thus Sfr. 17,000 under budget.
On the income side, the equal financial commitment of the federal authorities and Interpharma constitutes the basis for the Foundation's activities, each body donating a sum of Sfr. 445,000 to 3R.
Income totalled around Sfr. 897,000 (federal authorities and Interpharma together Sfr. 890,000, interest on bank account Sfr. 7,000) while total expenditure amounted to Sfr. 735,000, which gives a positive balance of approximately Sfr. 162,000. The balance of unused research funds therefore rose from around Sfr. 489,000 at the end of 2001 to Sfr. 651,000 at the end of 2002.
The budget for the year 2003 includes a sum of around Sfr. 707,000 for current projects and a maximum of Sfr. 550,000 for new projects.
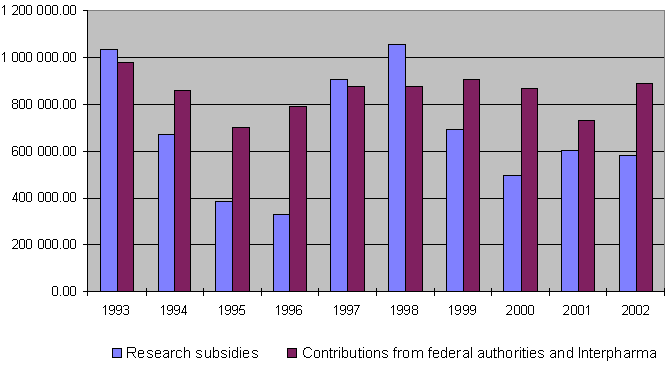
Overview of grants awarded between 1987 and 2002
By the end of 2002 a total of Sfr. 11,944,309.86 had been granted for projects and other subsidies, of which a total of Sfr. 10,638,121.31 has been paid out so far. Together the federal authorities and Interpharma have contributed Sfr. 12,616,000 to the Foundation since 1987.
10-year overview
7. Financial statements
| Profit and loss account 2002 | Expenditure | Income | |
|---|---|---|---|
| Income | |||
| Federal contribution | 445 000.00 | ||
| Contribution from Interpharma | 445 000.00 | ||
| Total contributions | 890 000.00 | ||
| Interest on bank account | 7 536.00 | ||
| Total income | 897 536.00 | ||
Expenditure | |||
| Research grants | 501 850.00 | ||
| Various 3R activities | 79 866.50 | ||
| Project supervision and information | 94 872.95 | ||
| Administrative expenses | 58 207.05 | ||
| Total expenditure | 734 796.50 | ||
| Excess income over expenditure | 162 739.50 | ||
| 897 536.00 | |||
| Balance as per 31st December 2002 | Assets | Liabilities | |
| Liquid Assets | |||
| Bank | 664 904.05 | ||
| Withholding tax | 5 745.10 | ||
| Accounting apportionment assets | 804.00 | ||
Liabilities | |||
| Accounting apportionment liabilities | 18 917.50 | ||
| Unused project funds | |||
| Carried forward 1. 1. 2002 | 488 796.15 | ||
| Excess income over expend | 162 739.50 | 651 535.65 | |
| Capital of the Foundation | 1 000.00 | ||
| 671 453.15 | 671 453.15 | ||
Contingent liabilities | |||
| Approved research grants not yet paid out | Sfr. 1 306 188.55. | ||
Münsingen, 18th March 2003
3R RESEARCH FOUNDATION
President: signed Dr. Hugo Wick
Secretary: signed E. Diener
8. Auditors' report to the Administrative Board
As 3R Research Foundation's auditors we have examined the books and the annual financial statements (balance sheet and profit and loss account) for the year ending 31st December 2002.
The Administrative Board is responsible for drawing up the financial statements while our task is to check and assess them. We hereby confirm that we are duly qualified to do this and that we have no vested interest in the Foundation.
We have checked the accounts and statements according to the generally accepted principles of accounting, by which this task must be planned and carried out in such a way that important errors in the financial statements are reasonably certain to be identified. We checked the entries and information in the statements in an analytical and investigative way using random samples. Furthermore, we assessed the implementation of standard accounting principles, the main evaluations and the presentation of the accounts as a whole. We are of the opinion that our examination provides a solid basis for our assessment of the accounts and statements of 3R.
In our opinion, the accounts have been kept and the financial statements have been drawn up according to the relevant laws and the statutes and regulations of the Foundation.
We therefore recommend that they be approved.
Gümligen, 12th March 2003
KPMG Fides Peat
signed Willy Beyeler, Cert. Auditor
signed Ursula Waber, Cert. Auditor
| Last modified 2014/06/04 |