 |
de | fr | en Druckansicht ![]()
3R-INFO-BULLETIN 17
May 2001
The Authors

The rabbit whole blood test was developed by Professor Dr. A. Wendel, PD Dr. Dr. T. Hartung and their team, in close collaboration with Prof. Dr. T.W. Jungi, Institute of Veterinary-Virology, University of Bern, Switzerland and Prof. Dr. R. Crameri, Swiss Institute for Allergy Research, Davos, Switzerland.
Professor Wendel is head of the Research Group Biomedical Pharmacology at the University of Konstanz, Germany. PD Dr. Dr. Hartung is leader of a subgroup.
e-mail: thomas.hartung@uni-konstanz.de
S. Schindler was mainly involved in the experiments and in the writing of this Bulletin.
As a veterinarian, she is in charge of the development of the rabbit IL-1ß ELISA as a doctoral thesis.
e-mail: stefanie.schindler@uni-konstanz.de
Dr. S. Fennrich is coordinator of the pyrogen team and contributed to the preliminary work, e.g. the immunization of the sheep and the cooperations with other institutes.
Ilona Kindinger and Heidrun Leisner are biologists, I. Seuffert and G. Pinksi are technicians all working in the pyrogen team.
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation
Fever in the test tube - towards a human(e) pyrogen test
Pyrogens induce the release of pyrogenic cytokines
Pyrogens are fever-inducing substances derived primarily from gram-negative and gram-positive bacteria. The guaranteed absence of pyrogens is a critical safety precaution for all drugs administered parenterally, since these contaminants can pose a life-threatening risk of shock to the patient.
Contact with minute concentrations of pyrogens - as low as 5 IU/kg, i.e. 500 pg/kg bodyweight - cause multiple reactions in the patient: human monocytes release several cytokines, the most important being IL-1ß, IL-6 and TNFa. The release of these cytokines can cause chills, rigors and hypotension. Furthermore, platelets can aggregate and the coagulation system become activated, resulting in disseminated intravascular coagulation and organ hypoxaemia, multiple organ failure and death by shock.
Human whole blood test (WBT) can replace animal test
Three tests are currently available to detect pyrogenic agents[*]. Of these, a commercially available test using human whole blood (PyroCheck) can detect a wide broad variety of pyrogens and is suitable for a broad range of applications (2,5,6; Table 1).
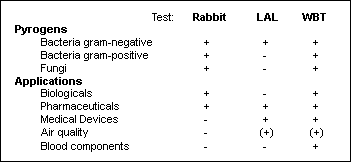
Table 1: Comparison of the three pyrogen tests (7)
Rabbit whole blood test: Bridging the gap between animal test and WBT
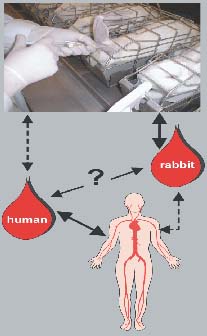 Fig. 1: Relationship between human and rabbit whole blood pyrogen test. |
Several steps were necessary to establish the rabbit whole blood test: i) Production of recombinant rabbit IL-1ß in E. coli (positive control for the ELISA, substance to immunise the animals in step two), ii) Immuni-sation of a sheep with the IL-1ß antigen, iii) Im-munisation of mice with IL-1ß and production of monoclonal antibodies, iv) Establishment of a sandwich ELISA (Enzyme Linked Immunosorbent Assay) with the antibodies and the antigen. These steps were successfully completed: recombinant IL1-ß was produced in a reliable quality and sufficient quantity. Monoclonal (mice) and polyclonal (sheep) antibodies against rabbit IL-1ß were isolated. The ELISA allows the quantitative determination of the endogenous rabbit pyrogen IL-1ß (Fig. 2).
Response to a pyrogen
The in vitro blood test allows the pyrogenic activity of various drugs and agents to be tested (Fig. 3), e.g. Pentaglobin. Pentaglobin is a biological drug which appears to cause excrutiating pain when administered intravenously in the live rabbit test. The rabbit whole blood test takes 24 hours to deliver results and requires only 100 μl of blood per sample. Currently (in contrast to the human WBT), fresh blood has to be used. About 7 ml of blood (i.e. enough for 70 samples) can be collected every two weeks from the ear vein of one rabbit without causing any harm to the animal.
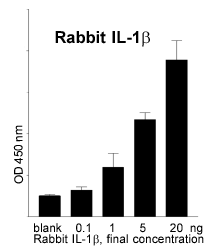 Fig. 2: Quantitative measure-ments of rabbit IL-ß with an ELISA. | 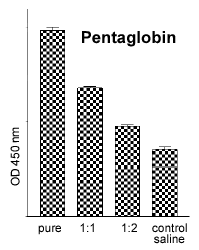 Fig. 3: TNFa release after incu-bation with a batch of penta-globin (pure = final concentra-tion: 10% ) and serial dilutions. |
A promising approach
The whole blood test in rabbits can help to explain false-positive and false-negative results when comparing the WBT with pyrogen test results in live rabbits. Further-more, pyrogen testing in animal blood makes it possible to examine species differences and test veterinary drugs in the target species. In the future, this approach could also help to avoid the use of putatively infectious human blood for pyrogen testing in vitro.
Published updated version of this Bulletin 17/2007 (PDF)
References:
- Fennrich, S., Fischer, M., Hartung,T., Lexa, P., Montag-Lessing,T., Sonntag, H.-G., Weigandt, M., Wendel,A. (1999): Detection of Endotoxins and other pyrogens using human whole blood. In: Brown F., Hendriksen, C., Sesardic D. (eds): Alternatives to animals in the development and control of biological products for human and veterinary use. Dev. Biol. Stand. Basel, Karger, 1999, Vol 101, 131-139.
- Hartung, T., Wendel, A. (1996): Detection of Pyrogens using human whole blood. In Vitro Toxicology, 9, 353-359.
- Eperon, S. and Jungi, T.W. (1996) The use of human monocytoid lines as indicator of endotoxin, J. Immunol. Methods. 194, 121-129.
- Hartung, T., Crameri, R., Wendel, A. (1998): Entwicklung eines Pyrogentests mit Kaninchenblut, ALTEX 15, 17-18.
- Bonenberger, J., Diekmann, D., Fennrich, S., Fischer, M., Friedrich, A., Hansper, M., Hartung, T., Jahnke, J., Löwer, J., Montag, T., Petri, E., Sonntag, H.-G., Weigand, M., Wendel, A., Zucker, B. (2000): Pyrogentestung mit Vollblut. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz. Springer-Verlag 2000, 43, 525-533.
- Jahnke, M., Weigand, M., Sonntag, H.-G. (2000): Comparative testing for pyrogens in parenteral drugs using the human whole blood pyrogen test, the rabbit in vivo pyrogen test and the LAL test. European Journal of Parenteral Sciences 2000; 5(2):39-44.
- Fennrich, S., Wendel, A., Hartung, T. (1999): New applications of the Human Whole Blood Pyrogen Assay (PyroCheck). ALTEX 16, 146-149.
| [*] | Pyrogen Testing - 3 different methodsIn vivo Rabbit Test: Limulus Amoebocyte Lysate Test (LAL): Human whole blood test (WBT): |
| Letzte Änderung: 30.01.2008 |