
3R-INFO-BULLETIN 17
| | May 2001The Authors The rabbit whole blood test was developed by Professor Dr. A. Wendel, PD Dr. Dr. T. Hartung and their team, in close collaboration with Prof. Dr. T.W. Jungi, Institute of Veterinary-Virology, University of Bern, Switzerland and Prof. Dr. R. Crameri, Swiss Institute for Allergy Research, Davos, Switzerland. Professor Wendel is head of the Research Group Biomedical Pharmacology at the University of Konstanz, Germany. PD Dr. Dr. Hartung is leader of a subgroup.
e-mail: thomas.hartung@uni-konstanz.de S. Schindler was mainly involved in the experiments and in the writing of this Bulletin.
As a veterinarian, she is in charge of the development of the rabbit IL-1ß ELISA as a doctoral thesis.
e-mail: stefanie.schindler@uni-konstanz.de Dr. S. Fennrich is coordinator of the pyrogen team and contributed to the preliminary work, e.g. the immunization of the sheep and the cooperations with other institutes. Ilona Kindinger and Heidrun Leisner are biologists, I. Seuffert and G. Pinksi are technicians all working in the pyrogen team.
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation |
Fever in the test tube - towards a human(e) pyrogen test
In an initial project (27-92) supported by the 3R Research Foundation Switzerland, a test using human cell lines as indicator for pyrogens was established (3). Subsequently, the human whole blood pyrogen test was developed by Hartung and Wendel (2). The development of a rabbit whole blood test (4), supported by the 3R Research Foundation in the present project, is important to be able to understand any differences between the pyrogenic activity of a sample tested in the human whole blood test and the existing live rabbit test. The results suggest that the in vitro rabbit test represents the missing link in the replacement of in vivo pyrogen testing in rabbits with in vitro methods.
Pyrogens induce the release of pyrogenic cytokines
Pyrogens are fever-inducing substances derived primarily from gram-negative and gram-positive bacteria. The guaranteed absence of pyrogens is a critical safety precaution for all drugs administered parenterally, since these contaminants can pose a life-threatening risk of shock to the patient.
Contact with minute concentrations of pyrogens - as low as 5 IU/kg, i.e. 500 pg/kg bodyweight - cause multiple reactions in the patient: human monocytes release several cytokines, the most important being IL-1ß, IL-6 and TNFa. The release of these cytokines can cause chills, rigors and hypotension. Furthermore, platelets can aggregate and the coagulation system become activated, resulting in disseminated intravascular coagulation and organ hypoxaemia, multiple organ failure and death by shock.
Human whole blood test (WBT) can replace animal test
Three tests are currently available to detect pyrogenic agents[*]. Of these, a commercially available test using human whole blood (PyroCheck) can detect a wide broad variety of pyrogens and is suitable for a broad range of applications (2,5,6; Table 1).
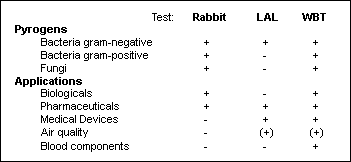
Table 1: Comparison of the three pyrogen tests (7)
Rabbit whole blood test: Bridging the gap between animal test and WBT
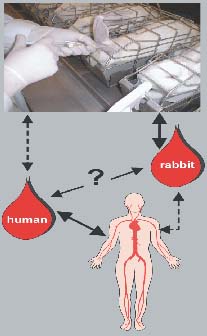
Fig. 1: Relationship between human and rabbit whole blood pyrogen test.
|
Unfortunately, the response to pyrogens in the WTB can be affected by the donor of the blood sample. In order to establish the validity of the WBT for the pharmacopoeia, it is important to be able to understand any differences between the pyrogenic activity in the human whole blood test and the existing live rabbit test and to be able to attribute these differences to species differences or to an aberrant response of the particular blood sample (Fig.1). As a link between the existing live test in rabbits and the WBT, the rabbit whole blood test was thus developed. This test uses the same species as the established in vivo test, but the same material and endpoint as the new in vitro test with human blood.Several steps were necessary to establish the rabbit whole blood test: i) Production of recombinant rabbit IL-1ß in E. coli (positive control for the ELISA, substance to immunise the animals in step two), ii) Immuni-sation of a sheep with the IL-1ß antigen, iii) Im-munisation of mice with IL-1ß and production of monoclonal antibodies, iv) Establishment of a sandwich ELISA (Enzyme Linked Immunosorbent Assay) with the antibodies and the antigen.
These steps were successfully completed: recombinant IL1-ß was produced in a reliable quality and sufficient quantity. Monoclonal (mice) and polyclonal (sheep) antibodies against rabbit IL-1ß were isolated. The ELISA allows the quantitative determination of the endogenous rabbit pyrogen IL-1ß (Fig. 2).
Response to a pyrogen
The in vitro blood test allows the pyrogenic activity of various drugs and agents to be tested (Fig. 3), e.g. Pentaglobin. Pentaglobin is a biological drug which appears to cause excrutiating pain when administered intravenously in the live rabbit test. The rabbit whole blood test takes 24 hours to deliver results and requires only 100 μl of blood per sample. Currently (in contrast to the human WBT), fresh blood has to be used. About 7 ml of blood (i.e. enough for 70 samples) can be collected every two weeks from the ear vein of one rabbit without causing any harm to the animal.
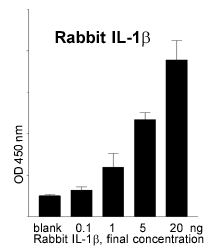
Fig. 2: Quantitative measure-ments of rabbit IL-ß with an ELISA. | 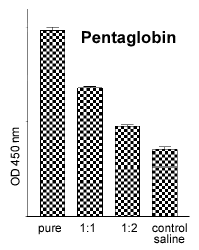
Fig. 3: TNFa release after incu-bation with a batch of penta-globin (pure = final concentra-tion: 10% ) and serial dilutions. |
A promising approach
The whole blood test in rabbits can help to explain false-positive and false-negative results when comparing the WBT with pyrogen test results in live rabbits. Further-more, pyrogen testing in animal blood makes it possible to examine species differences and test veterinary drugs in the target species. In the future, this approach could also help to avoid the use of putatively infectious human blood for pyrogen testing in vitro.
Published updated version of this Bulletin 17/2007 (PDF)
References:
- Fennrich, S., Fischer, M., Hartung,T., Lexa, P., Montag-Lessing,T., Sonntag, H.-G., Weigandt, M., Wendel,A. (1999): Detection of Endotoxins and other pyrogens using human whole blood. In: Brown F., Hendriksen, C., Sesardic D. (eds): Alternatives to animals in the development and control of biological products for human and veterinary use. Dev. Biol. Stand. Basel, Karger, 1999, Vol 101, 131-139.
- Hartung, T., Wendel, A. (1996): Detection of Pyrogens using human whole blood. In Vitro Toxicology, 9, 353-359.
- Eperon, S. and Jungi, T.W. (1996) The use of human monocytoid lines as indicator of endotoxin, J. Immunol. Methods. 194, 121-129.
- Hartung, T., Crameri, R., Wendel, A. (1998): Entwicklung eines Pyrogentests mit Kaninchenblut, ALTEX 15, 17-18.
- Bonenberger, J., Diekmann, D., Fennrich, S., Fischer, M., Friedrich, A., Hansper, M., Hartung, T., Jahnke, J., Löwer, J., Montag, T., Petri, E., Sonntag, H.-G., Weigand, M., Wendel, A., Zucker, B. (2000): Pyrogentestung mit Vollblut. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz. Springer-Verlag 2000, 43, 525-533.
- Jahnke, M., Weigand, M., Sonntag, H.-G. (2000): Comparative testing for pyrogens in parenteral drugs using the human whole blood pyrogen test, the rabbit in vivo pyrogen test and the LAL test. European Journal of Parenteral Sciences 2000; 5(2):39-44.
- Fennrich, S., Wendel, A., Hartung, T. (1999): New applications of the Human Whole Blood Pyrogen Assay (PyroCheck). ALTEX 16, 146-149.
| [*] | Pyrogen Testing - 3 different methodsIn vivo Rabbit Test:
The animals are injected i.v. with the drug and monitored for any reaction in the form of fever. This test is currently legally required by health authorities. The test, however, is subject to inherent problems, since the sensitivity of different species towards endotoxins varies by a factor of up to 10,000. Pyrogen testing currently requires about 200,000 rabbits each year world-wide. After a recuperation period of 2-3 weeks, the animals can be used again to test a new drug, providing that the test substances cause no permanent changes in the immune system of the rabbits. Limulus Amoebocyte Lysate Test (LAL):
This test measures the coagulation of the amoebocytes of the horseshoe crab, initiated by cell wall components (LPS) of gram-negative bacteria with a molecular weight of > 8000 daltons. The test cannot detect smaller LPS nor the LPS of gram-positive bacteria. Furthermore, the test cannot distinguish between the different types of endotoxins from gram-negative bacteria, which can vary in their fever-inducing potential in the mammal by a factor of 10,000 (1). Human whole blood test (WBT):
This test, using an ELISA, was developed in 1996 by Hartung and Wendel (2). It measures cytokine production, in this case IL-1ß, by human monocytes following a challenge with pyrogens. It is less expensive and more sensitive than the rabbit test and has the additional advantage of being able to examine the reaction strength directly in human material. Unlike the LAL, this test can detect not only endotoxins, but also lipoteichoic acids, fungi, and superantigens such as SEB (enterotoxin of staphylococcus aureus). |





