Figure 1: Human monocyte-derived microglia in culture.

Luis Filgueira
Department of Medicine, University of Fribourg, Switzerland
luis.filgueira@unifr.ch
Keywords: brain; immunology; neurology; validation
Duration: 1 year Project Completion: 2015
Background and Aim
Brain diseases are a burdensome problem for modern societies. Not surprisingly, therefore, substantial resources are invested in neuroscience research, which depends heavily on animal models. In this respect, microglia have assumed an important role. Animals are sacrificed for brain excision and the collection of cells of in-vitro studies. Animals are also used for in-vivo experiments. Depending on the aim of the study and on the nature of the procedure adopted, the in-vivo animal experiments involve different levels of invasiveness and suffering, which may include injuries to the brain and the spinal cord, if microglial response to trauma is being investigated, as well as exposure to toxic and infectious agents. Most of these experiments are conducted with human diseases in mind, even though animal microglia behave in many respects differently to human ones. The need to improve human in-vitro models of microglia is thus pressing.
Microglia are the unique resident immune cells of the brain. They play a crucial role in most brain diseases, being implicated in repair responses to injury, degeneration and infection-induced inflammatory reactivity. Being resident cells of the brain, they have to be isolated from fresh cerebral tissue for research purposes; only a few human microglial lines are available and for restricted applications only. We have developed a new human in-vitro model of microglia, which are derived from monocytes circulating in peripheral blood (Etemad et al. 2012). However, we now need to ascertain whether the properties of the human monocyte-derived microglia correspond to those of the brain-derived ones, which is a precondition for the validity of the in-vitro model.
Hypotheses:
Aims:
Method and Results
In progress (present status)
Conclusions and Relevance for 3R
It is expected that by validating our new in-vitro human model of monocyte-derived microglia, and by applying it to two important areas of brain research, namely Alzheimer’s disease and viral infection, the prototype will gain wide acceptance in the implicated research community and will be used to replace animal experiments. In consequence, we anticipate a dramatic reduction in the consumption of animals for research in the field of neuroscience.
References
Etemad, S., Mohd Zamin, R., Ruitenberg, M.J., and L. Filgueira, A novel human invitro microglia model: Characterization of human monocyte-derived microglia. J Neurosci Meth, 2012. 2009: p. 79-89.
Gentleman, S.M., Review: Microglia in protein aggregation disorders: friend or foe? Neuropath Appl Neurobiol, 2013. 39: p45-50.
Melief, J., Koning, N., Schuurman, K.G., Van de Garde, M.D.B., Smolders, J., Hoek, R.M., Van Eijk, M., Hamann, J., and I. Huitinga, Phenotyping primary human microglia: Tight regulation of LPS responsiveness. GLIA, 2012. 60: p 1506-1517.
Prabhakaran, P., Hassioutou, F., Blancafort, P., Filgueria, L., Cisplatin induces differentiation of breast cancer cells. Frontiers Oncol, 2013. 3:134 doi:10.3389/fonc.2013.00134.
Figures

Figure 1: Human monocyte-derived microglia in culture.
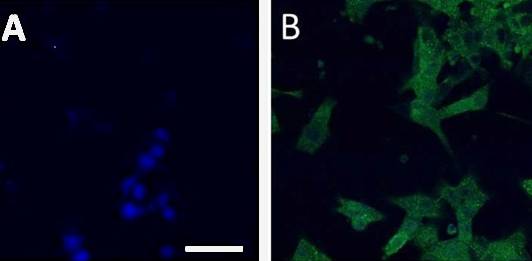
Figure 2: Expression of Iba1, a marker specific for microglia, by human monocyte-derived microglia. A) Staining control (blue nuclei stained with DAPI). B) Iba1 expressing cells are shown in green. The cells were treated with a monoclonal mouse anti-Iba1 antibody, and a secondary donkey anti-mouse antibody labelled with AlexaFlour 488.
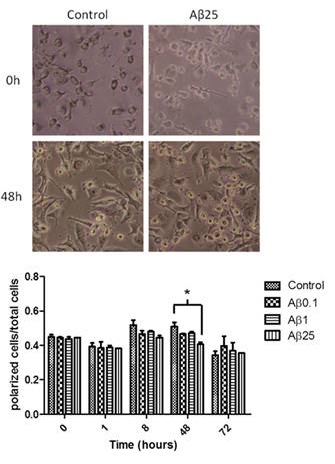
Figure 3: Influence of beta amyloid on human monocyte-derived microglia morphology. Left panel: light microscopy images of cells in culture before and after 48 hours of treatment with none or 25microgramm/ml of beta amyloid. Note the increased numbers of round shaped cells after 48 hours of treatment with beta amyloid indicating activation of microglia. Right panel: Quantification of activation of microglia after 0, 1, 8, 48 and 72 hours, after treatment with beta amyloid (0, 0.1, 1 and 25 microgramm7ml). There was a significant activation of the cells after 48 hours and treatment with 25microgramm of beta amyloid.
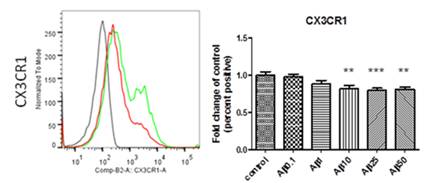
Figure 4: Down-regulation of expression of the chemokine receptor CX3CR1 in human monocyte-derived microglia after treatment with beta amyloid. Left panel: Representative histogram of flow cytometry measurements (black is staining control, green is CX3CR1 expression by control cells, red is expression of CX3CR1 after treatment with beta amyloid). Right panel: Quantitative analysis of CX3CR1 expression in cells treated with increasing concentrations of beta amyloid (0, 0.1, 1, 10, 25 and 50 microgramm/ml). n=4, ** is p<0.01, *** is p<0.005.