
3R-INFO-BULLETIN 12
| | September 1999The Author Dr. Felix Grimm* is head of a research and diagnostic working group at the Institute of Parasitology, University of Zürich, Switzerland Dr. Felix Grimm* is head of a research and diagnostic working group at the Institute of Parasitology, University of Zürich, Switzerland
current address:
* Institute of Parasitology,
University of Zürich,
Winterthurerstr. 266a,
CH-8057 Zürich,
Switzerland
felix.grimm@access.unizh.ch
EditorPeter Maier, Scientific Adviser of the 3R Research Foundation
|
Leishmaniasis: Development of an in vitro assay for drug screening
This project, supported by the 3R Research Foundation Switzerland, makes it
possible to culture the clinically relevant, intracellular stage of Leishmania,
the amastigote stage, in a medium without fetal calf serum and without host
cells. This allows a new in vitro drug screening assay for leishmanidicidal drugs
to be established and detailed studies on the mode of action of antileishmanial
drugs to be performed without the use of host animals.
Background
Leishmaniasis is a major parasitic disease in many tropical and subtropical countries[*]. Leishmania has a wide range of vertebrate hosts (e.g. rodents, dogs, humans) and are transmitted by insects of the genera Phlebotomus (Old World) and Lutzomyia (New World), also called sandflies or phlebotomine sandflies.
The parasites are transmitted as metacyclic flagellated promastigote forms (Fig.1a) by the bite of infected sandflies. After being taken up by phagocyting host cells (mainly macrophages), the promastigotes (spindle shaped, 10-15 μm, free flagellum) differentiate to amastigote forms (round to oval, 3-5 μm, lacking free flagellum, Fig.1b). These develop and multiply within the acidic phagolysosomal compartment of the host cells.
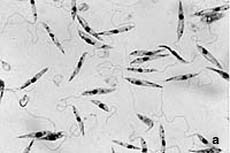 | 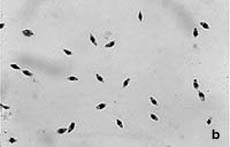 |
| Fig. 1. Promastigote (a) and amastigote (b) forms of Leishmania infantum. |
New leishmanicidal substances are required
A safe and effective vaccine is not yet available. Some currently used drugs cause severe adverse side effects; treatment failure is also common. A number of forms are resistant to conventional drug therapy, especially in HIV/leishmania coinfected patients in Africa. The development of new effective drugs is therefore an urgent task.
In vitro the promastigote stages can easily be cultivated. These forms have also been used for drug testing. However, since these test systems use the ‘wrong’ parasite stage (promastigote, insect stage instead of amastigote, vertebrate stage), the results do not always reflect the situation in the host and must be interpreted with caution (Fig. 2).
The 3R goals of the project
Test systems which rely on the ‘correct’ intracellular vertebrate (amastigote) stage of the parasite require either in vivo infection experiments using laboratory animals or time-consuming cocultivation of the parasite with macrophages or macrophage-like cells in vitro.
The goals of the present study are to cultivate amasti-gote forms of Leishmania species axenically (without host cells) in a medium free of fetal calf serum and to develop an in vitro screening assay for the identification of leishmanicidal drugs. This project fits well into the framework and goals supported by the 3R Research Foundation Switzerland. The project will lead to the replacement of animals during the initial step of drug development and to a reduction during the final assessment.
Developmental cycle of the parasite without the use of animals
Promastigote forms were propagated in vitro in a defined liquid medium free of serum or serum substitutes, at 27°C and a pH of 7.2 [1].
Amastigote forms were obtained from promastigotes by a stepwise increase in temperature to 36°C, followed by a stepwise decrease in pH of the medium to a final value of 5.4 [3]. They were cultivated in a slightly modified (MES-buffer, pH adjusted to 5.4) ‘promastigote’ medium [2] substituted with 1% of a commercially available serum fraction (FC III, HyClone) derived from abundantly available adult bovine serum.
By these procedures, parasites can be maintained by simulating their life cycle in culture flasks: switching from promastigote to amastigote conditions and back again. One cyle requires 3 to 4 weeks.
Reliable results with amastigote cultures
GlucantimeŽ is one of the most effective drugs used in the treatment of leishmaniasis. The sensitivity of the promastigote and the amastigote forms was compared. Only the amastigotes were sensitive to the drug, whereas the promastigotes were completely resistant (Fig. 2).
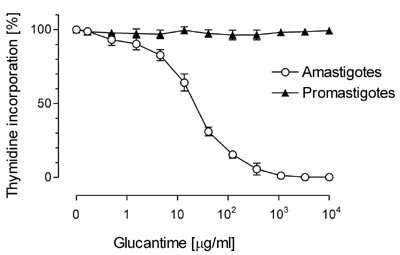 |
| Fig. 2. Comparison between amastigote and promastigote forms of L. infantum exposed to various concentrations of GlucantimeŽ. |
A new in vitro drug screening assay
The dose-response relationship of axenic amastigote forms of L. infantum was established after exposure to various drug concentrations for 48 hours. Inhibition of uptake of radiolabelled 3H-thymidine by the parasites was used as an indicator of the antileishmanial (cytostatic) activity. Typical dose response curves are given in Figure 3. The assay proved to be fast and reliable, and thus will allow the testing of large numbers of compounds within a short period of time.
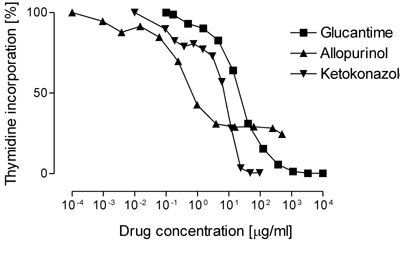 |
| Fig. 3. Dose response curves |
Studies of mechanisms of action
The possibility of producing large quantities of the clinically relevant amastigote stage in vitro allows detailed studies on the mode of action of antileishmanial drugs to be performed without the use of laboratory animals. Such studies will provide valuable information in the search for new leishmanicidal compounds.
Suggested tiered approach for drug development
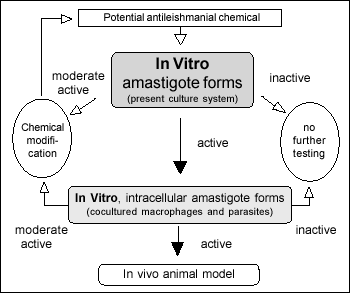
References:
- Kar K., Mekerji K., Naskar K., Bhattacharya A., Ghosh D.K. (1990): Leishmania donovani: A chemically defined medium suitable for cultivation and cloning of promastigotes and transformation of amastigotes to promastigotes.
J.Protozool. 37, 277-279. - Grimm F., Brun R., Jenni L. (1991): Promastigote infectivity in Leishmania infantum. Parasitol.Res. 77, 185-191.
- Eperon S. and McMahon-Pratt D. (1989): I. Extracellular cultivation and morphological characterization of amastigote-like forms of Leishmania panamensis and L.braziliensis. J. Protozool. 36, 502-510.
| [*] | Leishmaniasis
Three different forms of the disease are known in humans: cutaneous (CL, oriental sore), mucocutaneous (MCL, espundia) and visceral leishmaniasis (VL, kala-azar). In Europe, CL and VL are endemic around the Mediterranean basin (Portugal, Spain, south of France, Italy, Greece). In CL, cutaneous lesions or ulcerations are observed. These heal spontaneously (weeks to months) but leave the patient permanently scarred. MCL can lead to partial or total destruction of the mucous membranes of the nose, mouth and throat cavities and surrounding tissues. In VL, fever, hepatosplenomegaly and anaemia are the most prominent symptoms.
Endemicity is confirmed in 88 countries with an estimated total number of about 200-350 million people at risk. Annually, about 100’000 - 500’000 new cases of VL and 300’000 - 1.5 million new cases of CL or MCL are recorded. Leishmania is also recognized as an opportunistic pathogen of HIV-infected humans. |
 Dr. Felix Grimm* is head of a research and diagnostic working group at the Institute of Parasitology, University of Zürich, Switzerland
Dr. Felix Grimm* is head of a research and diagnostic working group at the Institute of Parasitology, University of Zürich, Switzerland




