 |
de | fr | en print view![]()
3R-Project 145-15
Combining computational modelling with in-vitro cellular responses in order to predict chemical impact on fish growth
Kristin Schirmer1 and Julita Stadnicka-Michalak2
1EAWAG, Swiss Federal Institute of Aquatic Science and Technology, 8600 Dübendorf, Switzerland;
2EPFL, Ecole Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland
Kristin.Schirmer@eawag.ch, Julita.Stadnicka-Michalak@epfl.ch
Keywords: fish; fish cell line; chemical risk assessment; toxicokinetic and toxicodynamic modelling; replacement
Duration: 2 years Project Completion: 2017
Background and Aim
We propose to develop an animal-free alternative to experiments with living fish to quantify the impact of chemicals on their early growth. Specifically, this project is based on the hypothesis that it should be possible to correlate the in-vitro cellular responses to various concentrations of these chemicals with their effects on the growth of different fish species over time. In particular, we postulate that by combining mathematical modelling with in-vitro cellular responses, it should be possible to assess the retardation of growth in juvenile fish without having recourse to living animals. We base our method on the assumption that the same chemical concentration in the internal organs of fish and in cultured cells would have the same impact on cell survival and cell population growth. In a former proof-of-principle investigation, cyproconazole and propiconazole were used as the reference chemicals, and the rainbow-trout gill cell-line, RTgill-W1, as a relevant in-vitro model (Figure 1; Stadnicka-Michalak et al., 2015). We now wish to further strengthen our approach by testing a wider variety of chemicals with the following specific aims:
- Prediction of the internal concentrations in fish to determine those that are needed for the in-vitro experiments.
- Measurement and prediction of the chemical toxicity in the in-vitro experiments using the RTgill-W1 cell-line.
- Scaling up of the in-vitro cellular responses to the chemical impact on fish.
- Extrapolation of the prediction to different fish species.
- Validation of the approach on the basis of independent measurements (no additional experiments with fish are needed).
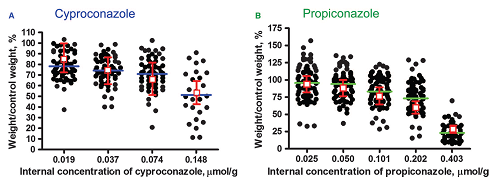
Figure 1: Proof-of-principle data revealing that a combination of RTgill-W1-cell-line responses and mathematical modelling can be used to predict reductions in fish growth. The black dots represent the measured reduction in fish weight, with each symbol representing one fish (“──” mean). The red squares represent the predictions that are based upon in-vitro cell-population growth data (including model uncertainties). For further details, see Stadnicka-Michalak et al., 2015.
Method and Results
The project will be carried out in four main steps. No animals will be needed in any of the performed experiments. The chemicals to be used in this project will be selected on the basis of their mechanisms of action on fish growth and their properties (e.g. volatility and hydrophobicity). Raw experimental fish-growth data will be gleaned from literature searches or obtained directly from companies/regulators).
STEP I: Prediction of the internal concentrations of the chemicals in fish using the Physiologically-Based Toxicokinetic (PBTK) model (Stadnicka et al., 2012): The PBTK-model will be applied to predict the chemical concentrations in various fish organs.
STEP II: Correlation of the chemical concentrations in fish with those in the in-vitro experiments: In-vitro experiments will be designed such that the concentrations in the cells should be the same as those in the tissues that are implicated in fish. The concentrations that are derived for various organs using the PBTK-model are taken as proxy for those that are needed in the cells in the in-vitro toxicity experiments (reverse toxicokinetics).
STEP III: Measurement and prediction of the chemical toxicity to fish cells: Fish growth depends on many factors, amongst which cell survival and proliferation play a dominant role. Thus, in this step, we will focus on measuring cell survival and proliferation after exposure to chemicals at the concentrations that will be established in STEP II.
STEP IV: Scaling up of the in-vitro cellular responses to the level of the whole organism: The cellular end-points ─ measured in STEP III ─ determine cell-population growth, which will be computationally correlated with the chemical impact on fish growth. We assume the total mass of all fish cells to be equivalent to fish weight. Hence the impact of cell-population growth on fish growth can be described by the von Bertalanffy growth model.
Conclusions and Relevance for 3R
Hundreds of thousands of juvenile fish are used annually to assess the influence of chemicals on their growth. To reduce this number, we propose a method which, for the first time, can quantitatively predict the impact of chemicals on fish growth from in-vitro data. This promising step towards alternatives to toxicity testing in fish is simple, cheap and rapid, and requires the use only of in-vitro data for model calibrations.
References
Stadnicka-Michalak J, Schirmer K, Ashauer R. (2015). Toxicology across scales: Cell population growth in vitro predicts reduced fish growth. Science Advances, Vol. 1, no. 7, e1500302, doi:10.1126/sciadv.1500302.
Stadnicka J, Schirmer K, Ashauer R. (2012). Predicting Concentrations of Organic Chemicals in Fish by Using Toxicokinetic Models. Environ. Sci. Technol. 46, 3273-3280.
| Last modified 2018/10/12 |