 |
de | fr | en print view![]()
3R-Project 143-15
An advanced in-vitro model of pulmonary inflammation based on a novel lung-on-chip technology
Olivier Guenat1 and Stefan Freigang2
1 ARTORG Center, University of Bern & Pneumology and Thoracic Surgery Clinics, University Hospital of Bern, Switzerland
2 Institute of Pathology, University of Bern, Switzerland
olivier.guenat@artorg.unibe.ch, stefan.freigang@pathology.unibe.ch
Keywords: lung; lung inflammation; mechanical stress; sepsis; chip; reduction; replacement
Duration: 1 year Project Completion: 2016
Background and Aim
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are important causes of morbidity and mortality in critically-ill patients. These life-threatening diseases are characterized by an accumulation of fluid in the alveolar sacs that ultimately prevents the diffusion of oxygen into the lung microvasculature. As an in-vivo model, lipopolysaccharide (LPS)-induced ALI is frequently used to study the molecular mechanisms that underlie, and potential therapies for inflammation-associated lung injury. However, since the experimental protocols are not standardized, inter-study comparisons are difficult. In addition, septic mice often suffer a high degree of distress during the experimental procedures. The development of an in-vitro system to accurately simulate the pathophysiological environment would, in serving as an alternative to the LPS-induced ALI-model in-vivo, help to cut down on the number of experiments with living animals.
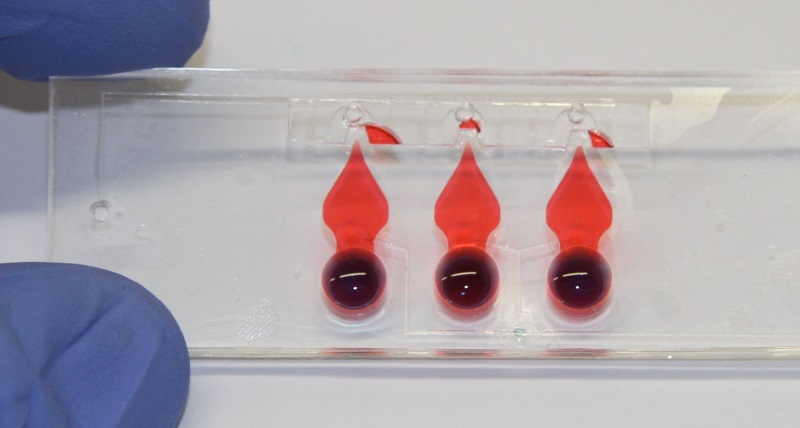
Figure 1: Lung-on-chip with three alveolar barriers. The thin, porous and flexible membrane can be cyclically stretched to mimic respiratory movements.
Method and Results
This project aims to establish an in-vitro model of pulmonary inflammation as an alternative to currently used in-vivo models of ALI. The in-vitro model is based on a recently developed lung-on-chip technology (Fig.1), which closely mimics the thin air-blood barrier as well as the cyclic mechanical stress that is imposed upon pulmonary tissue by respiration, and thus reproduces the lung parenchyma environment in an unprecedented way. On the chip, co-cultured primary murine lung epithelial and endothelial cells will be exposed to LPS, a potent microbial pro-inflammatory factor, which is known to induce sepsis and ALI. Key parameters of inflammation will be assessed in the in-vitro lung-on-chip model and the data directly compared to the responses that are elicited in the murine system.
Conclusions and Relevance for 3R
Apart from its major potential to replace animal studies, which are associated with severe distress, we expect that this novel technology will also permit a reduction in the overall number of studies with living mice. In particular, since a single chip contains several model alveoli, we anticipate that the data generated using each of these will substitute those that are gleaned from several individual research animals. Moreover, given the dimensions of the chip, sufficient primary lung endothelial and epithelial cells to seed several chips can be harvested from a limited number of donor animals, thereby promoting further reductions in mice usage.
References
- O. Stucki, J. D. Stucki, S. R. R. Hall, M. Felder, Y. Mermoud, R. a. Schmid, T. Geiser, and O. T. Guenat, A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip, 2015, 15, 1302–1310.
- P. Bretscher, J. Egger, A. Shamshiev, M. Trötzmüller, H. Köfeler, E.M. Carreira, M. Kopf, and S. Freigang, Phospholipid oxidation generates potent anti-inflammatory lipid mediators that mimic structurally related pro-resolving eicosanoids by activating Nrf2. EMBO Mol. Med., 2015, 7, 593–607.
- M. Felder, A. O. Stucki, J. D. Stucki, T. Geiser, and O. T. Guenat, The potential of microfluidic lung epithelial wounding: towards in-vivo-like alveolar microinjuries., Integr. Biol. (Camb)., 2014, 6, 1132–1140.
| Last modified 2018/10/12 |