
In 2015 the Foundation provided a total of Fr. 321,990.05 for 9 research projects that had been approved previously. Four projects were successfully completed and the Administrative Board approved 4 new projects. The latter, with a high level of relevance to the Foundation's 3R principles, were selected by the Evaluation Committee from the 45 project outlines originally submitted and were recommended for research grants. 3R Info Bulletins 54 and 55 included the results of two completed projects. The Confederation and Interpharma together provided Fr. 445,000. This is a lower amount than usual, owing to the fact that Interpharma contributed only Fr. 250,000 and the outstanding amount of Fr. 170,000, representing the shortfall to equal the amount provided by the Confederation in 2014, was not received and had to be written off. Since, in addition, Interpharma has also promised a maximum amount of Fr. 250,000 for 2016 and, in the future, all funds provided by the Confederation and Interpharma are to be used for the 3R Competence Centre, the Administrative Board was obliged to take the decision in December 2015 to forego calling for outline projects to be submitted for financial reasons.
On 1 July 2015 the Federal Council published its reply to proposition 12.3660 put forward by the National Council Science, Education and Culture Committee (SECC) on 17 August 2012 concerning "The future of the 3R Research Foundation and alternative methods in animal experimentation." The Federal Council’s reply put the focus on a new 3R Competence Centre with the aim of strengthening competence in this field. On 8 October 2015 the Foundation had the opportunity to present its point of view to the National Council SECC. Furthermore, the Foundation had to accept that it was unlikely to receive more funds for promoting research and that, for financial reasons, the 3R Research Foundation is unlikely to continue to exist alongside the planned 3R Competence Centre.
As a result, the Foundation will meet its present obligations in the immediate future but will not be able to allot any new research grants.
The 3Rs are Replace, Reduce and Refine animal experimentation. The 3Rs must be the guiding principles behind animal experimentation; if a study can be carried out without using any laboratory animals then such a procedure must be used. If it is essential to use laboratory animals under the terms of animal protection legislation the number used must be kept to a strict minimum. The third “R” requires that animals used for laboratory experiments be made to suffer an absolute minimum of pain and/or stress. The 3R Research Foundation funds research projects whose aim is to improve present-day experimental methods from the point of view of the 3Rs.
Detailed information about all the Foundation’s activities can be found on its website at www.forschung3r.ch.
A total amount of Fr. 321,990.05 was paid out for 9 ongoing projects during 2015.
Four new projects were approved in 2015 for which a total of Fr. 553,315.00 has been earmarked. These new projects are described in detail in the list of funded projects on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
Validation of a novel cell-based approach to study thyroidal physiology: Reduction and/or replacement of experiments with rodents (146-15) Dr. Gerasimos Sykiotis, Endocrinology, Diabetology and Metabolism Service, Vaud University Hospital, Lausanne. Rats are normally used to study the function of the thyroid and the role of its secreted hormones. Owing to the small size of the organ, such experiments involve the consumption of many animals. Using stem cells, it has recently become possible to generate three-dimensionally in vitro a functionally-competent model of the hormone-producing part of the thyroid, namely, the follicle. The project aims to validate this new in-vitro model using murine cells, and to ascertain whether various pharmacological and genetic manipulations influence its formation and signal transmission during hormonal steerage in a reproducible manner.
Combining computational modelling with in-vitro cellular responses in order to predict chemical impact on fish growth (145-15) Prof. Kristin Schirmer, EAWAG, Dübendorf. To examine the putatively negative side-effects of chemicals on the biological growth of fish and to determine the potentially poisonous impact of new substances that are intended for industrial and domestic use, hundreds of thousands of animals per year are legally subjected to experimental testing. The aim of this project is to develop a new computational model that is based upon toxicity data appertaining to cultured piscine cells. The comprehensive data-base that will be thereby derived will permit researchers in the future to conduct such testing computationally.
Development of in-vitro three-dimensional multi-cellular culture models to study the role of heterotypic cellular interactions in colorectal cancer invasion (144-15) Prof. Curzio Rüegg, Department of Medicine, Chair of Pathology, University of Fribourg. The survival chances of patients with cancer of the colon or the rectum are poor, owing primarily to the capacity of the cancerous cells to invade the surrounding tissue and to metastasize. Relatively little research has been conducted to elucidate the mechanisms that underlie the invasive process. The murine models that are currently available for studying the attributes of colorectal cancerous cells are of only limited use. Prof. Rüegg's research team has developed a promising new in-vitro model which can be used to investigate the interactions of the colorectal cancerous with stromal cells during the process of invasive growth. This model will now be rendered more sophisticated by including other cells that are implicated in the process (vascular cells, immune cells, etc.), as well as organ-like modules that better simulate cancer-cell biology.
An advanced in-vitro model of pulmonary inflammation based on a novel lung-on-chip technology (143-15) Prof. Olivier Guenat, ARTORG Centre, Lung Regeneration Tech, University of Berne. Inflammatory changes in lung tissue often lead to serious breathing problems. The complex processes that occur at the air-blood barrier, and which impair the exchange of gases and the flow of blood and influence the breathing mechanism, are normally simulated in animal experiments. The aim of this project is to create a lung model on a chip that will enable researchers to investigate various basic functions of the lung under the influence of pathological agencies, such as a post-traumatic inflammation.
Non-invasive electrical monitoring of the population spiking activity in the central nervous system (119-10) Dr. Sara Gonzalez Andino, Micro-circuit Neuroscience Laboratory, EPFL, Federal Institute of Technology, Lausanne. Electro-encephalography (EEG) measures electrical activity on the surface of the head. New EEG analysis models are being developed in order to precisely and topographically locate pathological processes as well as to improve our understanding of the importance and function of electrical brain activity. These new models are aimed at improving topographical resolution so that the electrical activity at even the topographical level of small nerve cell groups in the brain can be described. Experiments are being carried out to measure the electrical activity in the brain both through the skin and through the cranium with a high resolution using laboratory animals. Such experiments fall into category 3 with regard to suffering among the animals used. Dr. Gonzales Andino is proposing a new EEG analysis model that would enable such measurement to be made on the surface of the head. The principle of the new EEG model has been successfully tested and the results have been published. Further work must now be carried out to refine this method in order to achieve the aims defined at the outset.
Establishing a novel system for quantitative production of murine basophils in vitro (127-11) Prof. Thomas Kaufmann, Institute of Pharmacology, University of Berne. White blood corpuscles are a mixture of various types of cells that serve to protect the system against pathogens that enter the body. It would appear that a subpopulation of these cells – basophils – plays an important role in allergic reactions as well as in the modulation and regulation of immune responses. Research into the functioning of these cells normally involves murine basophils but, owing to their extremely low concentration in the blood (0.5% of white corpuscles), a large number of mice are required in order to be able to isolate minimum quantities of the cells. Prof. Kaufmann's research team has succeeded in producing immortalised basophil precursor cells in vitro which can provide functioning basophils in almost unlimited quantities.
Establishment of an in-vitro organ-slice defect model for meniscal repair in orthopaedic research (130-11) Prof. Ernst B. Hunziker, Centre of Regenerative Medicine for Skeletal Tissues, University of Berne. Injuries to the meniscus, especially in the human knee, are very frequent. Surgical removal of the damaged meniscus normally leads to arthritis in the joint after a few years. For this reason considerable efforts are being made to treat meniscus injuries biologically in order to avoid the later negative side-effects of surgical removal. Through tissue engineering using load-bearing material, regeneration cells and signal substances that regulate the healing processes, such ideas are normally tested on laboratory animals. Prof. Hunziker and his team succeded to establish a simple, cheap and standardised in-vitro meniscus model that would enable researchers to simulate the injury and the healing process in vitro on the basis of material obtained from slaughter houses (from bovine knee joints).
Development of a cardiovascular simulator with auto-regulation (134-12) Prof. Stijn Vandenberghe, ARTORG, Biomedical Research Centre, University of Berne. New materials and components such as heart valves, blood vessel walls, etc. are constantly being developed for heart surgery. The functioning, durability, tolerance, etc. of these components are tested on laboratory animals, which involves considerable suffering on their part. The aim of this project was to create an experimental cardiovascular machine which could be used for in vitro research and testing, including long-term testing. Prof. Vandenberghe's team has succeeded in building a realistic cardiovascular machine which accurately simulates the haemodynamic conditions and which can be regulated (using appropriate software) to simulate the various pathological circumstances.
3R-Info bulletins are published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html).
Establishment of a novel system for the production of large numbers of mouse basophils in vitro (no. 55, October 2015) Although basophils constitute only around 0.5% of the leukocyte population in the blood, they appear to play an important role in the body's allergic reactions as well as in modulating and regulating the immune response. Research into the function of these cells usually involves mouse basophils. Owing to the fact that their concentration in the blood is extremely small, many mice are required for isolating a minimal quantity of cells from the blood. Dr. Kaufmann's research team succeeded in producing immortalised basophil progenitor cells with which a virtually unlimited quantity of mature basophils can be obtained in vitro.
New tools for the generation of peptide and antibody-based ligands in vitro: Reduction of experimental animals for antibody production (no. 54, March 2015) The practical selection of specific antibodies for therapeutic, diagnostic and other purposes from a mixture of antibodies which are normally produced in animal experiments using repeated injections of immunogens is based on the use of phages (viruses) that are propagated in bacteria. They then express the desired target molecules on their surface (phage-display strategy), where they are recognised by the specific antibodies and then bind, thus enabling the researchers to select them. In this project, Prof. Heini and his team succeeded in developing an elegant, simplified phage-display strategy which requires fewer experimental steps and which can also be used in non-specialised laboratories. With this simplification and the possibility of the method being used more widely, the number of laboratory animals required can be considerably reduced.
The Foundation is a cooperative institution set up by the Parliamentary Group for Animal Experimentation Questions (public organ), Interpharma (association of pharmaceutical companies that carry out research in Switzerland; www.interpharma.ch/thema/uberinterpharma) and the Animalfree Research Foundation (animal protection). The Foundation was entered in the commercial register on 18 August, 1987.
The funds for subsidising research are provided principally by the Federal Food Safety and Veterinary Office and Interpharma.
The purpose of the 3R Research Foundation Switzerland is to promote alternative research methods through grants for research projects as well as to implement and promote the 3R principles. The organisation supports first and foremost projects aimed at developing new methods or refining accepted methods (validation) which offer improvements vis-à-vis standard animal experimentation in line with the 3R motto, Replace, Reduce, Refine.
A broad range of projects is funded on the condition that they are likely to replace animal experimentation or to reduce the number of animals used or the stress and/or pain suffered. Accordingly, projects based on the Foundation’s three principles and covering any of a broad selection of bio-medical disciplines will be taken into consideration.
The Administrative Board of the Foundation is made up of nine members, two representing the Swiss parliament, two representing animal protection, two from Interpharma and two from the Federal Food Safety and Veterinary Office, as well as a representative of other interested circles. Current members are:
Joachim Eder, member of the Council of States, Unterägeri (Chairman)
Dr. Peter Bossard, Horw (Deputy Chairman)
Dr. Philippe Bugnon, Institute of Laboratory Animal Science, University of Zurich
Dr. Isabelle Chevalley, member of the National Council, St. George (as from 26.5.15)
Dr. Kaspar Jörger, Federal Food Safety and Veterinary Office, Berne-Liebefeld
Dr. Ingrid Kohler, Federal Food Safety and Veterinary Office, Berne-Liebefeld
Dr. Birgit Ledermann, Novartis Pharma Ltd, Basle
Claudia Mertens, biologist, Zurich Animal Protection League, Winterthur
Nathalie Stieger, economist, F. Hoffmann-La Roche Ltd., Basle
Prof. Ernst B. Hunziker, University Hospital, Berne (Chairman)
Prof. Hans Acha-Orbea, Department of Biochemistry, University of Lausanne
Dr. Franziska Boess, F. Hoffmann-La Roche Ltd, Basle
Dr. Urban Deutsch, Theodor-Kocher-Institute, University of Berne
Dr. Robert Friis, University of Berne
Prof. Andrew Hemphill, Institute of Parasitology, University of Berne
Dr. Ingrid Kohler, Federal Food Safety and Veterinary Office, Berne-Liebefeld
Dr. Kurt Lingenhöhl, Novartis Pharma Ltd, Basle
Prof. Matthias Lutolf, Lausanne Federal Institute of Technology
Prof. Thomas Lutz, Institute of Veterinary Physiology, University of Zurich
Prof. Alex Odermatt, Department of Pharmaceutical Sciences, University of Basle
Prof. Tatiana Petrova, University of Lausanne
Prof. Barbara Rothen-Rutishauser, Adolphe Merkle Institute, University of Fribourg (as from 25.9.2015)
Dr. Stefanie Schindler, Animalfree Research Foundation, Berne
Prof. Ernst B. Hunziker, University Hospital, Bern
Ernst P. Diener, lawyer, Münsingen
DieWirtschaftsprüfer.ch AG, Thun
Federal Department of Home Affairs
In May 2015, the Administrative Board was pleased to appoint Dr. Isabelle Chevalley, a member of the National Council for the Green Liberal Party, from St. George, as the second parliamentary representative on the Board.
At the beginning of the year, Prof. Marianne Geiser Kamber from the Anatomical Institute of the University of Berne resigned from the Evaluation Committee. The Administrative Board thanked her most sincerely for her many years of work on the Committee. Her seat has been taken up by Prof. Barbara Rothen-Rutishauser from the Adolphe Merkle Institute of the University of Fribourg, whose special field is diseases of the respiratory system.
In the Foundation’s twenty-ningth year of existence the Administrative Board met three times, namely in May, September and December, for a half-day meeting. Apart from the statutory business concerning the end of the business year 2014, the Board addressed the following issues.
In May, the Board focused on the financial statements for 2014 and earmarking research funds for ongoing projects. In addition, it took note of the final reports on four completed projects. The appointment of Dr. Isabelle Chevalley, a member of the National Council, to the Administrative Board means that the second parliamentary seat has now been filled.
At its meeting in September, the focus was on funding for new projects. Out of a total of 9 project proposals selected by the Evaluation Committee from the 45 applications received, 4 were finally approved. The Administrative Board noted the Federal Council's reply dated 1 July 2015 to the proposal put forward by the National Council Science, Education and Culture Committee (SECC) on "The future of the 3R Research Foundation and alternative methods in animal experimentation." The representative of the Federal Food Safety and Veterinary Office informed the Board of the plans to set up a new 3R Competence Centre. One option that remains to be examined would be for the 3R Research Foundation to modify its official purpose and become legal entity for the 3R Competence Centre.
At the Administrative Board’s December meeting, Prof. Hans Wyss, the Director of the Federal Food Safety and Veterinary Office, informed the Board about the plans and conditions for the creation of the 3R Competence Centre. It then became clear that, with its current purpose and scope of activities, there would be no future for the 3R Research Foundation. Bearing this in mind, the Administrative Board examined the overall financial situation in detail. It concluded that the existing funds would suffice to meet all current obligations vis-à-vis research projects but that no further obligations could be entered into. For this reason it was decided not to call for project proposals for 2016. The meeting finished with a review of activities during 2015 and those planned for 2016, which was followed by way of a thank-you for the work carried out in 2015 by a dinner for the whole Board.
Under the chairmanship of the Scientific Advisor, the Evaluation Committee held two meetings during the year, where in particular they examined 45 new applications for funding of which they chose 9 likely projects; finally 4 were approved. In addition they examined the final reports on a total of four completed projects and submitted them to the Administrative Board. We should like to take this opportunity to thank the members of the Evaluation Committee for their voluntary work in this connection.

During 2015 four projects were completed (119-10, 127-11, 130-11, 134-12). Together with those projects completed earlier, this brings the total of finished projects to 132 out of 146.
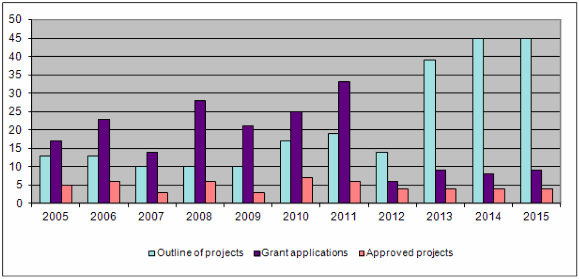
The bar-chart shows that the trend in the number of project outlines, detailed proposals and project approvals remained similar to the previous year. The total number of project outlines and detailed proposals received each year has almost tripled over the past 10 years. The number of project outlines and applications for funding shown above from 2013 on cannot be compared with figures for previous years since a new two-level application procedure was introduced in 2013. First, applicants submit project outlines which are assessed by the Evaluation Committee in the first round. Only those applicants putting forward a project with clear relevance to the Foundation's 3R principles are subsequently invited to submit a detailed project proposal.
Forty-five project outlines were submitted. After examining them all, the Evaluation Committee asked 9 applicants to submit a detailed proposal, and out of the 9 detailed proposals the Administrative Board finally approved 4 for a research grant. Regardless of the many applications received, there is little fluctuation in the number of projects approved for funding owing to the limited means available to the Foundation (funding rate <10%).
Research funding for the 9 ongoing projects amounted to Fr. 321,990.05 in 2015. The sum of Fr. 7,517.20 was used for an interactive internet platform as well as for participation in meetings where the projects funded by the 3R Foundation were presented. Together with expenditure on project supervision (Fr. 93,134.47) and provisions for project funding (Fr. 187,538.90 made up of Fr. 587,322.80 for provisions for 2016 minus Fr. 399,783.90 for provisions for 2015), total expenditure for research projects amounted to Fr. 610,180.62. Administrative costs totalled Fr. 92,992.35. Total expenditure therefore amounted to Fr. 703,172.97.
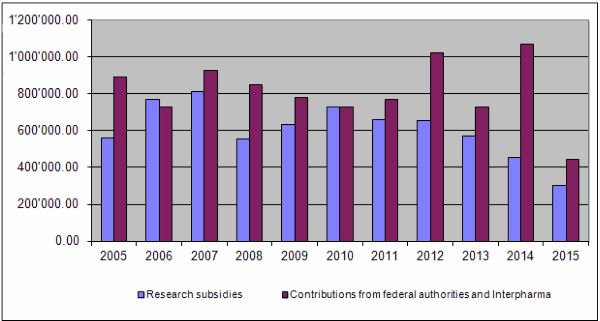
On the income side, the equal financial commitment of the Confederation and Interpharma has thus far constituted the basis for the Foundation's activities. In 2015 the Federal Food Safety and Veterinary Office provided the Foundation with Fr. 365,000. Interpharma, however, transferred the sum of Fr. 250,000 and informed the Board that an equal amount was the maximum that it could promise for 2016. This meant that the outstanding amount of Fr. 170,000 compared with the Confederation's contribution in 2014, which had been booked on the assets side as an accounting apportionment item, had to be written off. This resulted in a net contribution of Fr. 80,000 from Interpharma for 2015. Together with other income (Fr. 46.50) and a repayment of unused funding (Fr. 24,782.75), total income for 2015 amounted to Fr. 469.829,25.
The balance therefore shows an excess of expenditure over income of Fr. 233,343.72. This amount has been covered on the balance sheet by a transfer from capital funds. Consequently, capital funds or unused research funding was reduced from Fr. 258,604.89 at the end of 2014 to Fr. 25,261.17 at the end of 2015.
At the end of 2015 the sum earmarked by the Administrative Board in principle on the basis of project approvals but as yet not paid out amounted to Fr. 851,481.45. Out of this, Fr. 587,322.80 is covered by provisions. Consequently, as at 31.12.2015, there remained contingency commitments in the amount of Fr. 264,158.65 that are not shown in the financial statements. The Foundation's credit balance with Interpharma arising from its Commitment VI amounted to Fr. 2,132,000.00 at the end of 2015.
The budget for 2016 includes Fr. 583,322.80 for current projects.
Together the federal authorities and Interpharma have contributed Fr. 23,211,000.00 to the Foundation since 1987. At the end of 2015 a total of Fr. 19,628,418.65 had been granted for projects and other subsidies, of which Fr. 18,776,937.20 had been paid out so far. Expenditure for project evaluation and supervision amounted to Fr. 2,252,041.48 and the accumulated administrative costs totalled Fr. 1,958,929.17 (8.3% of total expenditure or 10% of grants paid).
The noticeable difference between contributions for 2014 and 2015 can be explained by the fact that the outstanding amount of Fr. 170,000.00 owed by Interpharma to bring its contribution into line with that from the Confederation in 2014 was not received. It had already been booked as income in 2014 and was included on the assets side as an accounting apportionment item. It had to be written off in 2015. This resulted in the reduced contribution for 2015.
| Profit and loss account | 2015 | 2014 |
|---|---|---|
| Federal contribution | 365 000.00 | 535 000.00 |
| Interpharma contribution | 80 000.00 | 535 000.00 |
| Contributions to the Foundation | 445 000.00 | 1 070 000.00 |
| Research grants | -329 507.25 | -454 511.05 |
| Reimboursement of research grants | 24 782.75 | 0.00 |
| Adjustment reserves for research grants | -187 538.90 | -399 783.90 |
| Project supervision and information | -93 134.47 | -94 923.85 |
| Balance for current projects | -140 397.87 | 120 781.20 |
| Administrative costs | -92 992.35 | -113 717.50 |
| Intermediate balance | -233 390.22 | 7 063.70 |
| Financial income | 46.50 | 179.83 |
| Financial result | 46.50 | 179.83 |
| Allocation to capital funds | -7 243.53 | |
| Withdrawal from capital funds | 233 343.72 | |
| Balance | 0.00 | 0.00 |
| Balance as per 31st December | 2015 | 2014 |
| Assets | ||
| Liquid Assets | 616 992.37 | 509 678.94 |
| Accounts payable | ||
| Accounting apportionment assets | 2 367.60 | 171 019.30 |
| Current assets | 619 359.97 | 680 698.24 |
Liabilities | ||
| Accounting apportionment liabilities | 5 776.00 | 21 309.45 |
| Reserves for research grants | 587 322.80 | 399 783.90 |
| Borrowed capital | 593 098.80 | 421 093.35 |
| Capital | ||
| – Carried forward 1 January | 258 604.89 | 251 361.36 |
| – Change in capital | -233 343.72 | 7 243.53 |
| Balance as at 31 December | 25 261.17 | 258 604.89 |
| Foundation's capital | 1 000.00 | 1 000.00 |
| Organisational capital | 26 261.17 | 259 604.89 |
| 619 359.97 | 680 698.24 | |
Contingent liabilities
Approved research grants not yet paid out Fr.. 264,158.65.
Münsingen, 15 March 2016
3R RESEARCH FOUNDATION
The Chairman: signed Joachim Eder
The Administrator: signed Ernst P. Diener
DieWirtschaftsprüfer.ch AG in Thun audited the financial statements for the year according to standards of limited auditing and did not find any indication that the accounts and statements do not correspond to current legislation or the principles and regulations of the Foundation.