
In 2012 the Foundation provided a total of Fr. 618,767.15 for 14 research projects. The Confederation and Interpharma made a total contribution of Fr. 1,020,000.00. The Administrative Board approved 4 new projects, while 4 projects were successfully completed. In view of the limited funds available, the call for projects was restricted to one deadline only, namely in February 2012. Furthermore, a new 2-stage application procedure was introduced. Out of the 13 project proposals submitted the Evaluation Committee selected 6 that were more relevant to the 3R principles and asked those applicants to submit a detailed description of their projects. A further selection process eliminated two projects whose relevance to the 3R principles was less evident, there being insufficient funds in the Foundation’s coffers for all six. The 3R Info Bulletins 48 - 50, which were circulated to around 1,000 readers, included the results of two completed projects. On the 25th anniversary of the creation of the Foundation, some thoughts about the activities and the future role of the Foundation were also presented.
To mark its 25th anniversary in 2012, the Foundation organised a scientific workshop together with the Swiss Laboratory Animal Science Association, and with the collaboration of the Swiss Toxicology Association, in November in the Technopark in Zurich. During this workshop, ecopa (European Consensus Platform for 3R Alternatives to Animal Experimentation) held its annual general meeting. The workshop was attended by 400 specialists in the field of animal experimentation and alternative methods.
The future direction of the Foundation’s activities was the focus of further debate among the Administrative Board. In May the Foundation had the opportunity to give a presentation to the National Council’s Committee for Science, Education and Culture in which it described developments in animal experimentation. The Scientific Advisor, Prof. Peter Maier, retired at the end of the year. The Administrative Board offered him their very best wishes for the future, and were lucky enough to be able to replace him by Prof. Ernst B. Hunziker from the University of Berne, who is a proven specialist in his field.
The 3Rs are Replace, Reduce and Refine animal experimentation. The 3Rs must be the guiding principles behind animal experimentation; if a study can be carried out without using any laboratory animals then such a procedure must be used. If it is essential to use laboratory animals under the terms of animal protection legislation the number used must be kept to a strict minimum. The third “R” requires that animals used for laboratory experiments be made to suffer an absolute minimum of pain and/or stress. The 3R Research Foundation funds research projects whose aim is to improve present-day experimental methods from the point of view of the 3Rs.
Detailed information about all the Foundation’s activities can be found on its website at www.forschung3r.ch.
Two more propositions for 3R-relevant methods were added to the section entitled “3R-Methods“.
Experimental colitis through serial assessment of disease activity by 18F-FDG PET/CT Dr. Dominik Bettenworth, University Clinic Münster, Germany. Preclinical evaluation of potential drug candidates for human inflammatory bowel disease is performed in murine models of colitis. The most widely used model is dextran-sodium sulfate(DSS)-induced colitis. Non-invasive imaging of murine experimental colitis by Positron emission tomography (PET) combined with radiotracers is a feasible alternative and offers the opportunity of serial follow-up investigations. This technique significantly reduces the number of test animals required.
Quantitative, non-destructive biofilm analysis on irregular surfaces using microcalorimetry Dr. Martin Clauss, Orthopaedics Department, Cantonal Hospital, Liestal und Dr. Andrej Trampuz, University Hospital, Lausanne, Switzerland. This in-vitro approach allows the quantification of biofilm formation on implants, human bone grafts and bone-graft substitutes by means of microcalorimetry. The method has the advantage of allowing the detection of bacterial biomass without removing the biofilm from the surface of the specimen.
A total amount of Fr. 618,767.15 was paid out for 14 ongoing projects during 2012.
Four new projects were approved in 2012 for which a total of Fr. 472 039.00 has been earmarked. These new projects are described in detail in the list of funded projects on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
Antibody phage selection strategy for application in non-specialized laboratories (131/12) Prof. Christian Heinis, Laboratory of Therapeutic Peptides and Proteins, EPFL, Swiss Federal Institute of Technology Lausanne, Switzerland. Monoclonal antibodies for use in research continue to be produced using hundreds of thousands of rodents. The main reason for this is the complexity of the in vitro method and the limited use of commercially available phage libraries. In this project a new combination of techniques will be examined (high-throughput sequencing and DNA synthesis) with which the procedure can be carried out at a much lower cost. A sufficiently complex phage library should be made available to research laboratories without any restrictions and phage selection should be possible using fewer experimental steps. The simplified procedure could also be carried out in non-specialized laboratories. As a result, the simplification of antibody phage selection could help achieve a breakthrough in the application of this in vitro method, the principle of which has already been known to researchers for a number of years.
Identification of predictive in vitro markers for hematopoietic stem cell function (132/12) Prof. Matthias P. Lutolf, Institute of Bioengineering, EPFL, Swiss Federal Institute of Technology Lausanne, Switzerland. In order to test whether hematopoietic stem cells (HSC) are capable of producing normal blood cells over a longer period of time, they are normally implanted into mice. Measurements are then made to see whether the HSC are able to reconstitute the blood system that has been destroyed through radiation. This procedure causes extreme suffering to the animals used and a large number of mice have to be sacrificed. In this project, Prof. Lutolf and his team intend to identify new cell markers to predict the long-term functioning of HSC that have been developed in vitro.
Development of an in vitro system to grow and investigate vascular endothelial cells under physiological flow (133/12) Prof. Robert Rieben, Department of Clinical Research, University of Berne, Switzerland. The function of endothelial cells in the walls of blood vessels is often investigated in vivo, causing suffering to the laboratory animals involved. Conventional in vitro procedures simulate the physiological conditions with inadequate accuracy. This new in vitro experimental method is aimed at replacing many such in vivo studies. In the proposed in vitro model the pressure and shearing power of the pulsating blood, for example, are simulated. This system should make it possible to find answers to questions concerning ischaemia, reperfusion and transplantation that have so far been investigated in in vivo experiments involving considerable pain and suffering for the laboratory animals concerned.
Development of a cardiovascular simulator with autoregulation (134/12) Prof. Stijn Vandenberghe, ARTORG Center for Biomedical Research, University of Berne, Switzerland. Many studies are carried out in vivo for testing and gaining regulatory approval for cardiological devices. The aim of this project is to adapt a cardiovascular simulator to in vivo conditions in such a way that far fewer laboratory animals are required for testing such devices as blood-pumps, for example. The challenge in this case is to simulate self-regulating, clinically relevant mechanisms such as baroreflex (blood pressure and heart beat regulation) and the Frank-Starling mechanism (correlation between inflow and expulsion). The (preliminary) testing of devices using such further developed simulators should help to ensure that only the most promising devices are tested in vivo.
Engineering of a human brain tumor model to replace animal experimentation (115/09) Prof. Olivier Preynat-Seauve, Department of Pathology and Immunology, University Medical Centre, University of Geneva, Switzerland. The aim of this project was to combine the delicate cell culture models developed by the project leader for brain tissue (engineered neural tissue, ENT, from embryonic stem cells) and glioblastomas (engineered glial tumors, EGT, from human tumor tissue), and thus to develop an in vitro system for studying glioblastomas and characterising their molecular structure. Such a system would replace in vivo methods that cause considerable suffering to the laboratory animals used, in particular in relation to the intracerebral or subcutaneous injection of human tumor cells in immuno-suppressed mice. The team were successful in establishing their in vitro system. Histo-pathological and molecular-biological studies were carried out on the reaction of the EGT transplanted into the ENT. It now remains to examine the various ways in which this model can be used.
Development of non-invasive strategies to study spinal cord disease, injury and repair (120/10) Prof. Denis Jabaudon, Neuroscience Department, University Medical Centre, University of Geneva, Switzerland. In this project, Prof. Jabaudon aimed to develop a method for the non-invasive injection of substances (e.g. plasmids) into the spinal cord of mice without using a surgical procedure. Such a method would result in a lower rate of mortality and less suffering among the laboratory animals used. The research team was able to demonstrate that the high-resolution ultrasound method for positioning the hypodermic needle for cortical injections was equally as accurate as the stereotactic method. On the other hand, the ultrasound method proved to be unsuitable for spinal injections. It was therefore not possible to establish the non-invasive injection method for use in the spinal cord.
A new in vitro model to study therapeutic approaches to improve spinal cord regeneration and repair after injury (121/10) Prof. Roman Chrast, Department of Medical Genetics, University of Lausanne, Switzerland. This project aimed to examine the effect of various substances on spinal cord regeneration in vitro using mouse organotypic longitudinal, sagital slice cultures developed by the project leaders. The research team was also able to induce lesions similar to those seen in multiple sclerosis, thus allowing for functional and structural studies. Using this method it would be possible to avoid a large number of tests on live animals. Comparative in vivo and in vitro studies using potential new medicines remain to be carried out.
Use of "moribund" stage in the acute fish toxicity test according to OECD guideline 203 and its effect on LC50 values (123/10) Dr. Hans Rufli, ecotoxsolutions, Basle, Switzerland. The aim of this project is to define the final ”moribund” stage in the acute toxicity test in fish in order to introduce it as a criterion for interrupting the test under OECD guideline 203. Retrospective analysis of data and protocols from hundreds of toxicity tests carried out on fish has shown that by introducing the “moribund” criterion the suffering of the fish through the acute effects of a test substance could be reduced by up to 92 hours thanks to its earlier removal from the test. By introducing the “moribund” criterion, the LC50 value could be lowered, on average by a factor of 2, in up to half of the experiments. Attempts are now being made, in collaboration with the Swiss Federal Office for the Environment and various EU countries, to get the OECD protocol changed. This would help to achieve a significant reduction in the level of suffering among the fish used in this test.
3R-Info bulletins are published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html).
A novel ex vivo mouse aorta perfusion model (no. 48, February 2012) In this project, Prof. Patrick Hunziker, Clinic for Intensive Medicine, University of Basle, Switzerland and his research group have extracted and cultured ex vivo aorta tissue of transgenic (ApoE-/-) mice. A method was developed for obtaining the tissue and its subsequent on-line observation under a fluorescence microscope. It was possible to identify and characterise the sclerotic sections of the walls of the aorta (plaques) following perfusion of the aorta with specific markers. The time taken for changes to the cells could also be determined. The results obtained corresponded to a great extent to findings from studies using ApoE-/- mice, which shows that many studies, for example for preselecting potential new medicines, could be carried out ex vivo.
Bacterial Meningitis: Investigating Injury and Regenerative Therapy in vitro (no. 49, June 2012) Bacterial meningitis (BM) causes brain injury in the dentate gyrus (DG) of the hippocampus, with bacterial components and the host organ's inflammatory reaction contributing jointly to the insult. The researchers, Prof. Stephen Leib and team from the Neuroinfection Laboratory at the Department of Clinical Research, University of Berne, Switzerland, succeeded in differentiating neural stem cells and precursor cells at different stages of development and simulating in them the reaction to the damaging effect of bacterial meningitis. It is likely that compromised brain areas can be repaired by grafting suitable stem/progenitor cells into brain tissue. This process has been successfully carried out in cultured brain slices in vitro. Thanks to the in vitro methods developed by the researcher, it will be possible in the future to carry out indicative preliminary examinations that are necessary to identify appropriate cells for transplantation, for example. It would only be necessary to use laboratory animals for the final confirmation of the in vitro findings.
Still relevant twenty-five years on; 25 years of Funding the 3Rs (no. 50, December 2012). Prof. Peter Maier, Scientific Advisor of the 3R Research Foundation Switzerland until the end of 2012, reflects on the visibility, activities and future role of the Foundation. Dr. Stefanie Schindler, an animal welfare expert, assesses the Foundation’s twenty-five years of funding. To mark the Foundation’s 25th anniversary, the two authors describe the present situation with regard to animal experimentation in Switzerland (awareness of the Foundation’s work, limited funds) and the progress achieved so far concerning reduction und refinement. In order to prevent an increase in the use of laboratory animals in the field of life sciences, the established research promotion involving live animals should specifically support aspects relevant to the 3R principles. A study is under way to analyse what the 3R Research Foundation has achieved over the past 25 years in various fields through its albeit limited funding; initial results are published.
The Foundation is a cooperative institution set up by the Parliamentary Group for Animal Experimentation Questions (public organ), Interpharma (association of pharmaceutical companies that carry out research, comprising at present Actelion Ltd, Merck Serono Ltd, Novartis Pharma Ltd, F. Hoffmann-La Roche Ltd, and the associated members Bayer (Switzerland) Ltd, Cilag Ltd and Vifor Ltd) and the Animalfree Research Foundation (animal protection). The Foundation was entered in the commercial register on 18 August, 1987.
The funds provided to subsidise research stem from the Federal Veterinary Office and Interpharma.
The purpose of the 3R Research Foundation Switzerland is to promote alternative research methods through grants for research projects as well as to implement and promote the 3R principles. The organisation supports first and foremost projects aimed at developing new methods or refining accepted methods (validation) which offer improvements vis-à-vis standard animal experimentation in line with the 3R motto, Replace, Reduce, Refine.
A broad range of projects is funded on the condition that they are likely to replace animal experimentation or to reduce the number of animals used or the stress and/or pain suffered. Accordingly, projects based on the Foundation’s three principles and covering any of a broad selection of bio-medical disciplines will be taken into consideration.
The Administrative Board of the Foundation is made up of nine members, two representing the Swiss parliament (1 seat vacant), two representing animal protection, two from Interpharma and two from the Federal Veterinary Office, as well as a representative of other interested circles. Current members are:
Christine Egerszegi, member of the Council of States, Mellingen (Chairwoman)
Dr. Peter Bossard, Horw (Deputy Chairman)
Dr. Franz P. Gruber, Doerenkamp-Zbinden Foundation, Küsnacht (until 31. 12. 2012)
Dr. Ingrid Kohler, Federal Veterinary Office, Berne-Liebefeld
Silvia Matile-Steiner, lawyer, Reinach
Dr. Markus Schmutz, Novartis Pharma AG, Basle (as from 30.3.2011)
Nathalie Stieger, economist, F. Hoffmann-La Roche Ltd., Basle
Prof. Hans Wyss, Director of the Federal Veterinary Office, Berne-Liebefeld
Prof. Peter Maier, University of Zurich (until 31. 12. 2012) (Chairman)
Prof. Ernst B. Hunziker, University of Berne (as from 1. 3. 2013)
Dr. Franziska Boess, F. Hoffmann-La Roche Ltd, Basle
Prof. Clemens A. Dahinden, Institute of Immunology and Allergology, University Hospital, Berne
Prof. Marianne Geiser Kamber, Institute of Anatomy, University of Berne
Prof. Andrew Hemphill, Institute of Parasitology, University of Berne
Prof. Simon P. Hoerstrup, Swiss Center for Regenerative Medicine (SCRM),
University and University Hospital Zurich (as from 17.1.2012)
Dr. Ingrid Kohler, Federal Veterinary Office, Berne-Liebefeld
Dr. Kurt Lingenhöhl, Novartis Pharma Ltd, Basle
Prof. Thomas Lutz, Institute of Veterinary Physiology, University of Zurich
Dr. Martin Reist, Sanisys Ltd, Biel (until 30. 4. 2013)
Dr. Stefanie Schindler, Animalfree Research Foundation, Berne
Prof. Peter Maier, University of Zurich (until 31. 12. 2012)
Prof. Ernst B. Hunziker, University of Berne (as from 1. 3. 2013)
Ernst P. Diener, lawyer, Münsingen
Waber Treuhand GmbH, Einigen
Federal Department of Home Affairs
Prof. Simon P. Hoerstrup, head of the Swiss Centre for Regenerative Medicine at Zurich University Hospital and the University of Zurich, was elected to the Evaluation Committee.
As the representative of the animal protection lobby, Dr. Franz P. Gruber resigned from the Administrative Board having reached the age limit of 70 and was warmly thanked for all his work on behalf of the Foundation. Since joining the Board in 1995, Dr. Gruber had constantly brought the animal protection point of view to bear among the Board members and, with his comprehensive knowledge of research activities in relation to the 3R principles, often provided valuable impulses for the decisions that were taken. The Administrative Board greatly appreciated his willingness and his unflagging commitment. He was replaced in May 2013 by Claudia Mertens, a biologist who works with the Zurich Animal Protection League and is now the representative of this field on the Board.
At the end of the year, Prof. Peter Maier resigned as the Foundation’s Scientific Advisor. He was also thanked most sincerely for all the work he had done over the past 12 years and for establishing a scientifically solid and recognised funding system through the 3R Foundation. Prof. Ernst. B. Hunziker from the University of Berne has been appointed as the new Scientific Advisor and head of the Evaluation Committee.
In the Foundation’s twenty-sixth year of existence the Administrative Board met five times, namely in January, March, August, October and December, for a half-day meeting. Apart from the statutory business concerning the end of the business year 2011, the Board addressed the following issues.
In January, the Board discussed personnel issues and the future direction of the Foundation’s activities. At its March meeting it focused on the financial statements for 2011 and the earmarking of research funds for ongoing projects. In addition, discussions covered ways of instigating collaboration with the Swiss National Science Foundation (SNF) in order to persuade the latter to provide funds for research relevant to the 3R principles. The Administrative Board would like to see increased efforts in networking in order to achieve greater application of the 3R principles in scientific research.
At its meeting in August, the focus was on funding for new projects. Out of a total of 13 proposed projects, 4 were finally approved. The final reports on 4 completed projects were received. A decision was then taken regarding the procedure for advertising for a replacement for the Scientific Advisor, who would retire at the end of 2012. Finally, the Administrative Board was informed about how preparations for the 25th anniversary workshop were progressing; the event was to be organised together with the Swiss Laboratory Animal Science Association and would take place in Zurich on 19 and 20 November 2012. Information on the Foundation’s 25th anniversary would be provided to the media and would be used to inform the general public about our commitment to animal protection and science. A number of successful projects would be presented in easily comprehensible terms to provide an insight into the Foundation’s work and the research teams that receive funding.
The Administrative Board met again in October in order to discuss the applications for the position of Scientific Advisor; a decision was taken as to which applicants should be called in for interviews. Candidates for the position each gave a presentation at the Administrative Board’s December meeting and a decision was taken. Dr. Franz P. Gruber, who has been a member of the Board, and Prof. Peter Maier, the retiring Scientific Advisor, were warmly thanked for their hard work and commitment on behalf of the Foundation. Any other business included a discussion of the year’s activities with the members of the Evaluation Committee, the 25th anniversary workshop and the talks with representatives of the SNF, which unfortunately proved unfruitful. The evening ended with a dinner.
The Strategy Committee set up by the Administrative Board held various meetings at which it drew up proposals for the Foundation’s future activities.
The Administrator was busier than usual during 2012 with five meetings and preparations for changes in personnel. The Administrator deals with all matters concerning the Foundation that cannot be passed on to anyone else. In particular, he prepares all the necessary information for the Administrative Board to take their decisions, as well as dealing with correspondence with applicants and project managers. He also deals with payments, book-keeping, closing the books at the end of the financial year and the budget. In addition, he prepares the Annual Report as well as texts for the Foundation’s website.
Under the chairmanship of the Scientific Advisor, the Evaluation Committee held two meetings during the year, where in particular they examined 13 new applications for funding of which they chose 6 likely projects; finally 4 were approved. In addition they examined the final reports on 4 completed projects and submitted them to the Administrative Board. We should like to take this opportunity to thank the members of the Evaluation Committee for their voluntary work in this connection.
The Scientific Advisor's tasks included publishing the 3R Info Bulletins (in the form of brochures and on the Foundation’s website at www.forschung3r.ch), writing the brief scientific reports in English which present the projects receiving funding on the Foundation’s website and regularly updating these reports. He was also kept busy – as always – advising applicants and project managers, obtaining intermediate reports, evaluating project outlines, dealing with enquiries and explaining why projects had been rejected. Finally, he represented the Foundation at several scientific meetings in Switzerland and abroad, namely at the 1st European Conference on Replacement, Reduction and Refinement of Animal Experiments in Ecotoxicology at the EAWAG in Dübendorf. Within the context of the European Partnership for Alternative Approaches to Animal Testing (EPAA), the Scientific Advisor, being a member of the Mirror Group and the Scientific Advisory Committee, played a consulting role in relation to this European initiative.
Furthermore, in 2012 much of the Scientific Advisor’s time was spent preparing and organising, together with the Swiss Laboratory Animal Science Association, the Foundation’s 25th anniversary workshop, which was held on 19 and 20 November at the Technopark in Zurich. The Administrative Board would like to thank him most warmly for all his work in this connection; the participants were also immensely appreciative of the excellent programme and the impeccable organisation of the meeting.
The workshop was held on 19 and 20 November 2012 at the Technopark in Zurich. There were over 400 participants, of which some 100 attended the 3R sessions. During the 5 sessions, 22 speakers from Switzerland and abroad described the many possible 3R-relevant methods that can be used in various fields. The Swiss Toxicology Association held one session in which they presented 3R-relevant progress in the field of toxicology. Representatives of the European Consensus Platform for 3R Alternatives (ecopa) from eight European countries provided information about where and what kind of 3R-relevant research was being funded and what issues were being discussed at present by the various EU committees. Short presentations were used to explain the different opportunities of consensus (interaction and sponsoring) between the 4 partners (the state, scientific circles, animal protection organisations and industry) in Europe.
The 111 pages of the Proceedings include general information about the meeting and the welcome addresses given by the Chairwoman of the 3R Research Foundation and the President of the SGV, and were handed over to all the participants. The main body of the Proceedings, however, comprises the 30 papers presented and summaries of the 18 on-going projects funded by the Foundation. The presentations included a total of over 130 images. The Proceedings and press releases about the workshop can be consulted on the Foundation’s website.
At the dinner held at the Hotel St. Gotthard in Zurich, Prof. Peter Maier, who organised the event, welcomed the 53 diners, in particular the speakers, representatives of the Federal Veterinary Office and Interpharma, and current and former members of the Administrative Board and the Evaluation Committee. In a short speech he described the Foundation’s activities over the past 25 years. His hard work, which resulted in an extremely successful event, is much appreciated.

2012 four projects were completed (115-09, 120-10, 121-10 and 123-10). Together with those projects completed earlier, this brings the total of finished projects to 117 out of 134.
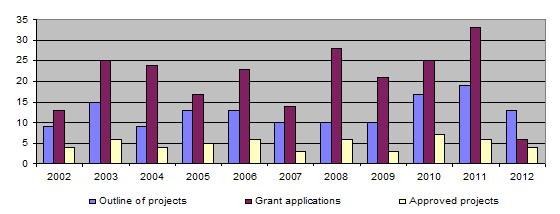
The bar-chart shows that the number of project outlines and applications increased considerably up to 2011. In contrast, the number of projects approved has remained more or less unchanged, owing to the Foundation’s financial limitations. In 2012, in view of the limited funds available, the call for projects was restricted to one deadline only, namely in February 2012, and a new two-stage application procedure was introduced. Initially, applicants submit a project proposal, 13 such proposals being received in 2012. After the proposals have been examined by the Evaluation Committee, the latter then asks those whose proposed projects are relevant to the 3R principles to draw up detailed applications, 6 such detailed descriptions being submitted in 2012. Insofar as there was only one deadline for submitting proposals, which then underwent a preliminary triage by the Evaluation Committee, the figures for 2012 concerning proposals and full applications cannot be compared with figures for previous years.
A total of some Fr. 676,848.25 was paid out for research in 2012.
Operational expenditure for 2012 amounted to Fr. 242,919.51 (project monitoring and information Fr. 107,417.44, administrative costs including office infrastructure Fr. 135,502.07). The increase in operational expenditure was mainly due to the activities surrounding the Foundation’s 25th anniversary and for the appointment of the new Scientific Advisor. Total expenditure therefore amounted to Fr. 919,767.76. Total expenditure therefore amounted to Fr. 919,767.76.
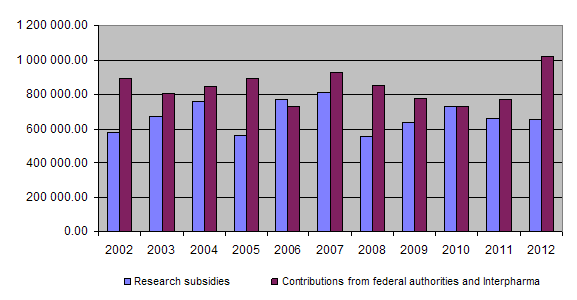
On the income side, the equal financial commitment of the federal authorities and Interpharma represented the basic funding for the Foundation’s activities, each providing in 2012 an amount of Fr. 485,000. This annual contribution was higher than usual and helped to alleviate the Foundation’s financial problems somewhat. In addition they made a one-time supplementary contribution of Fr. 50,000 for the Schindler Assessment Project. A further income item of Fr. 23,109.86 resulted from financial income and repayment of unused project funding.
Total income was therefore Fr. 1,043,109.86 while total expenditure amounted to Fr. 919,767.76, giving an excess of income over expenditure of Fr. 123,342.10. The unused funds item consequently rose by Fr. 164,528.86 to Fr. 287,870.96 by the end of 2012, the latter figure constituting the Foundation’s new reserve of liquid assets.
At the end of 2012 the total earmarked for projects approved by the Board but not yet paid out amounted to Fr. 844,157.15. This future liability is covered by Interpharma’s promise of funding (V) together with the contribution from the Confederation. The Foundation’s credit with Interpharma amounted to Fr. 712,000 at the end of 2012.
The budget for 2013 includes Fr. 603,534.15 for current projects and a maximum amount of Fr. 500,000 for new projects.
At the end of 2012 a total of Fr. 18,289,696.45 had been granted for projects and other subsidies, of which Fr. 17,445,539.30 had been paid out so far. Together the federal authorities and Interpharma have contributed Fr. 20,966,000.00 to the Foundation since 1987.
| Profit and loss account 2012 | Expenditure | Income | |
|---|---|---|---|
| Income | |||
| Federal contribution | 495 000.00 | ||
| Contribution from Interpharma | 525 000.00 | ||
| Total contributions | 1 020 000.00 | ||
| Capital yield | 409.60 | ||
| Reimbursement of research grants | 22 288.76 | ||
| Other income | 511.50 | ||
| Total income | 1 043 109.86 | ||
Expenditure | |||
| Research grants | 676 848.25 | ||
| Project supervision and information | 107 417.44 | ||
| Administrative expenses | 135 502.07 | ||
| Total expenditure | 919 767.76 | ||
| Excess income over expenditure | 123 342.10 | ||
| 1 043 109.86 | |||
| Balance as per 31st December 2012 | Assets | Liabilities | |
| Liquid Assets | |||
| Bank | 195 032.36 | ||
| Accounts payable | 354.50 | ||
| Accounting apportionment assets | 124 044.00 | ||
Liabilities | |||
| Accounting apportionment liabilities | 30 559.90 | ||
| Unused research funds | |||
| Carried forward 1. 1. 2012 | 164 528.86 | ||
| Excess income over expend | 123 342.10 | 287 870.96 | |
| Capital | 1 000.00 | ||
| 319 430.86 | 319 430.86 | ||
Contingent liabilities | |||
| Approved research grants not yet paid out | Sfr. 844 157.15. | ||
Münsingen, 15 March 2013
3R RESEARCH FOUNDATION
Chairwoman: signed Christine Egerszegi
Secretary: signed Ernst P. Diener
Waber Treuhand GmbH in Einigen audited the financial statements for the year according to standards of limited auditing and did not find any indication that the accounts and statements do not correspond to current legislation or the principles and regulations of the Foundation.