 |
de | fr | en print view![]()
Annual report 2010
The 3R Research Foundation in 2010
In 2010 the Foundation provided a total of Fr. 728,600 for 17 research projects. The Confederation and Interpharma each made a contribution of Fr. 365,000. The Administrative Board approved 7 new projects, while 3 projects were successfully completed; 18 applications were rejected. The 3R-Info-Bulletins 42 - 44, which were circulated to around 1,000 readers, included the results of three of the completed projects. The final report on the results of the EU Start-up project (Scientific and technological issues in 3R alternatives research in the process of drug development and union politics), to which the Foundation contributed in 2008 through a meeting of experts in Basle chaired by the Scientific Adviser, is now available. The Administrative Board made efforts to network with other institutions and organisations in order to achieve greater awareness of the 3R principles. It is not yet possible to identify concrete results of these efforts.
3R-Principles
The 3Rs are Replace, Reduce and Refine animal experimentation. The 3Rs must be the guiding principles behind animal experimentation; if a study can be carried out without using any laboratory animals then such a procedure must be used. If it is essential to use laboratory animals under the terms of animal protection legislation the number used must be kept to a strict minimum. The third “R” requires that animals used for laboratory experiments be made to suffer an absolute minimum of pain and/or stress. The 3R Research Foundation funds research projects whose aim is to improve present-day experimental methods from the point of view of the 3Rs.
Summary of the Year’s Activities
The Foundation’s website
Detailed information about all the Foundation’s activities can be found on its website at www.forschung3r.ch. A new addition to the website is a section entitled “3R-Methods“, aimed at raising awareness regarding selected 3R methods intended to resolve specific problems in the field of life sciences.
17 projects subsidised
A total amount of Fr. 728,615.96 was paid out for 14 ongoing projects and 3 that were completed during 2010.
Seven new projects
Seven new projects were approved in 2010 for which a total of Fr. 660,330 was earmarked. These new projects are described in detail in the list of funded projects on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
Engineering of an in vitro hepatocyte tissue system for malaria liver infection research (118/10) Dr. Dalu Mancama, CSIR, Biosciences Division, Pretoria, South Africa. Resistance to currently available drugs has led to a need to develop new products or vaccines. During the development of the disease, the interaction between parasites (Plasmodia) and host in the liver plays an important role. Plasmodium falciparum, which is responsible for malaria in humans, cannot be investigated using animal models. For this reason, surrogate animal models are used because of the lack of human liver cell models in which the interaction between parasites and host can be studied. A novel tissue culture system (3D cultures) has been developed to increase the infectiousness of Plasmodium f. in human liver cells. If this is successful it will be possible to develop new treatments in liver cell cultures without the need to use laboratory animals.
Non-invasive electrical monitoring of the population spiking activitiy in the central nervous system (119/10) Dr. Sara Gonzalez Andino, Department of Clinical Neuroscience, University of Geneva, Switzerland. Various non-invasive methods (fMRI, optical imaging) are currently used for neuro/electrophysiological studies of the brain. None of these methods enables researchers to determine electrical activity in single cells or cell populations in the CNS, however. To date it has been necessary to implant electrodes in order to obtain more precise measurements. On the basis of the latest data from studies of epileptic patients and primates it is expected that a correlation will be found between scalp EEG and spiking activity (changes in the action potential) in cell populations. By comparing existing data series using appropriate software it should be possible to increase the spatial resolution of EEGs and to carry out many studies directly on humans. This should lead to a reduction in the number of primates required for invasive neurological investigations.
Development of non-invasive strategies to study spinal cord disease, injury and repair (120/10) Prof. Denis Jabaudon, Department of Basic Neurosciences, University of Geneva, Switzerland. Worldwide, spinal cord injury and neurodegenerative diseases (e.g. amyotrophic lateral sclerosis) are the focus of intense and increasing research using laboratory animals with the aim of determining the processes involved and devising possible therapies. Such experiments using rats and mice often necessitate spinal cord intervention. These surgical procedures are time-consuming and cause a good deal of suffering for the animals; in addition, mortality is high (up to 30%). Many such surgical procedures could be replaced by the percutaneous introduction of a micropipette, using high-resolution ultrasound guidance, to inject dyes, genetic material or cells or for therapeutic intervention or placing electrodes. This procedure takes only 2-3 minutes and does not involve exposing the spinal cord. Consequently there is far less stress for the animal and the reduced mortality rate would mean that fewer animals would be needed.
A new in vitro model to study therapeutic approaches to improve spinal cord regeneration and repair after injury (121/10) Prof. Dr. Roman Chrast, Department of Medical Genetics, University of Lausanne, and Prof. Dr. Josef Kapfhammer, Institute of Anatomy, University of Basle, Switzerland. Spinal cord injuries (SCI) affect millions of people throughout the world every year, resulting in paraplegia and chronic pain. Until now animal models have been used to study therapeutic approaches to regeneration and repair. Such studies cause a high level of distress for the animals and the complexity of the in vivo processes reduces the reproducibility of the results. Using organotypic cultures of spinal cord slices that have been developed recently, it is possible to create lesions similar to SCI. The aim of this project is to characterise the function of the cells in the slice and to test the regenerative ability of two substances already used in clinical treatment, namely Nogo A and Cethrin. If the researchers succeed in achieving comparable results to those obtained in in vivo studies it will be possible to do away with a large number of experiments using laboratory animals.
Improved perioperative analgesia and reduced stress during recovery for the experimental animal: ultrasound-guided sciatic and femoral nerve block in sheep and quantitative assessment of block quality (122/10) Dr. Helene Rohrbach, Department of Clinical Veterinary Medicine, University of Berne, Switzerland. Sheep are widely used for orthopaedic studies. In many cases the practicability (dose, duration of effect, method of administration) of the usual post-operative analgesic protocols is questionable, since sheep show hardly any outward signs of pain. In order to be sure of blocking pain more successfully, anaesthetics are administered regionally using a perineural catheter. The placing of the catheter near the sciatic and femoral nerves is monitored via ultra-sound, similar to the procedure used in humans. The efficacy of the analgesic is quantified. This should lead to the successful blockade of pain using lower doses and with fewer side-effects.
Use of "moribund" stage in the fish acute toxicity test according to OECD guideline 203 and its effect on LC50 values (123/10) Dr. Hans Rufli, ecotoxsolutions, Basle, Switzerland. In the fish acute toxicity test according to OECD guideline 203, LC50 is assessed in terms of the concentration of a test substance at which 50% of the fish die within an exposure period of 96 hours. In this project, sub-lethal effects on fish during the first 48 hours taken from reports on past testing are analysed and compared retrospectively to findings after 96 hours. The aim here is to be able to define the “moribund” stage whereby fish can be removed from the test at an earlier point without affecting the results. This would mean that the fish would suffer from the effects of the test substance concentrations for a shorter time.
Comparative in vitro and in vivo testing on biofilm formation on the surface of bone grafts (124/10) Dr. Martin Clauss, Orthopaedic Department, Cantonal Hospital Liestal, Switzerland. Infections associated with orthopaedic implants are difficult to treat because the resistant micro-organisms spread around the implant in a biofilm. For this reason it is important to develop materials that prevent the formation of this biofilm. These characteristics are being tested in guinea pigs. The research team has already developed an in vitro system and its practicability will now be examined in a comparative study of the guinea pig test and the in vitro test.
Three projects successfully completed
Development of a novel multicellular 3-dimensional blood brain barrier in vitro model (93/04) Dr. Omolara Ogunshola, Institute of Veterinary Physiology, Vetsuisse Faculty, University of Zurich. In this project a 3-dimensional model of the blood-brain barrier was simulated using endothelial cells, astrocytes and pericytes. In a collagen matrix the cells form a unit with a synergistic effect. The physiological reaction of this unit to various noxious substances was comparable to that seen in experiments involving live animals. This method therefore enables researchers to carry out mechanistic experiments on the function of the blood-brain barrier without using laboratory animals. The results obtained are summarised in 3R-Info-Bulletin no. 42.
Development of a three-dimensional enteric cell culture model for in vitro studies of the intestinal eukaryotic parasites Cryptosporidium spp. (97/05) Prof. Alexander Mathis, Institute of Parasitology, University of Zurich. Until now pathogenic intestinal parasites have been isolated in host animals (new-born calves) for subsequent experiments. In order to replace the need for live animals, a rotating system was used to culture epithelial enteric cells as a substrate for producing Cryptosporidium parvum parasites. The cell cultures were successfully established and can be considered as an in vitro model of the intestinal epithelium. The rate of production of the parasites in the cell cultures was extremely low, however, and limited to two days.
Establishment of an in vitro system for the prediction of the degree of virulence of classical swine fever virus isolates (105/06) Dr. Nicolas Ruggli, Institute of Virology and Immunoprophylaxis (IVI), Mittelhäusern, Switzerland. The degree of virulence of the swine fever virus after an outbreak is normally tested on pigs. This project involves developing a procedure for determining the virulence of the swine fever virus in cell cultures. Dr. Nicolas Ruggli and his research team succeeded in finding a cell-line in which the 17 selected, well documented virus isolates reproduced using a standard culture method without causing changes to the cell-line. The characteristics of the viruses in three different cell tests (cell-line, swine macrophages and plasmocytic dendritic cells) reliably showed whether the viruses had a high, medium, low or negligible level of virulence. Thanks to this in vitro diagnostic method, it will no longer be necessary to test for virulence in live animals. The results of this project were summarised in 3R-Info-Bulletin no. 44.
3R-Info-Bulletins
3R-Info bulletins are published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html).
The blood-brain barrier in a dish: a new multicellular in vitro model (no. 42, February 2010) The research team led by Dr. Lara Ogunshola, Institute of Veterinary Physiology, University of Zurich, succeeded in creating an in vitro 3-D model of the blood-brain barrier comprising three main cell types: epithelial cells, astrocytes and pericytes. The blood-brain barrier protects the brain but its function is affected by various diseases such as stroke, Alzheimer’s and neuro-AIDS. The physiological reaction to hypoxic conditions corresponded to the in vivo situation. The team was able to investigate complex processes such as the synergistic interactions between various cell types. This method will make it possible to answer numerous questions concerning the function of the blood-brain barrier without resorting to the use of laboratory animals.
Fish acute toxicity test: The number of animals can be reduced (no. 43, June 2010) In testing fish for acute toxicity (OECD Protocol no. 203), the dosage of the substance tested is often too high or too low. Together with Swiss and foreign specialists, Dr. Hans Rufli of Ecotoxsolutions in Basle analysed historical data on hundreds of chemicals used in agriculture and industry. Using retrospective computer simulations of testing procedures the team were able to demonstrate that the number of fish involved could be reduced by 15%. If the preliminary tests are carried out on fish embryos, a further considerable reduction in the number of fish required can be achieved without compromising the validity of the results with regard to the toxicity of potential environmental pollutants. The necessary steps have already been taken to have these results taken into account in the OECD guidelines.
From pigs to cells: Virulence of classical swine fever virus is predictable in cell cultures (no. 44, October 2010). Dr. Nicolas Ruggli and his research team at the Institute for Viral Diseases and Immunoprophylaxis, Mittelhäusern, Switzerland, have succeeded in developing a cell culture test for assessing the virulence of the virus in classical swine fever. The degree of virulence is important for monitoring and preventing the disease. Until now it could be assessed only by examining infected animals. The tests involve a cell culture for reproducing the virus and three tests to determine viral foci on a cell overlay, the macrophage infection rate and the production of IFN-alpha. A comparison with the virulence of 17 known viruses showed that, taken together, the three parameters provide a prediction of virulence that is equally as good as that obtained using live animals.
1. Origin of the Foundation
The Foundation is a cooperative institution set up by the Parliamentary Group for Animal Experimentation Questions (public organ), Interpharma (association of pharmaceutical companies that carry out research, comprising at present Actelion Ltd, Merck Serono Ltd, Novartis Pharma Ltd, F. Hoffmann-La Roche Ltd, and the associated members Bayer (Switzerland) Ltd, Cilag Ltd and Vifor Ltd) and the Animalfree Research Foundation (animal protection). The Foundation was entered in the commercial register on 18 August, 1987.
The funds provided to subsidise research stem from the Federal Veterinary Office and Interpharma.
2. Purpose of the Foundation
The purpose of the 3R Research Foundation Switzerland is to promote alternative research methods which avoid the use of animals, through grants for research projects. The organisation supports first and foremost projects aimed at developing new methods or refining accepted methods (validation) which offer practical improvements vis-à-vis standard animal experimentation in line with the 3R motto, Replace, Reduce, Refine.
A broad range of projects is sponsored on the condition that they are likely to replace animal experimentation, to reduce the number of animals used or the stress and/or pain suffered. Accordingly, projects based on the Foundation’s three principles and covering any of a broad selection of bio-medical disciplines will be taken into consideration.
3. Organisation of the Foundation
The Administrative Board
The Administrative Board of the Foundation is made up of nine members, three representing the Parliament (2 seats vacant), two representing animal protection, two from Interpharma and two from the Federal Veterinary Office. Current members are:
Christine Egerszegi-Obrist, member of the Council of States, Mellingen (Chairwoman)
Dr. Peter Bossard, Animalfree Research Foundation, Zurich (Vice-Chairman)
Chantal Galladé, National Councillor, Winterthur (until 31.12.2010)
Dr. Franz P. Gruber, Doerenkamp-Zbinden Foundation, Küsnacht
Prof. Paul Herrling, Head of Research, Novartis International, Basle (until 31.12.2010)
Dr. Ingrid Kohler, Federal Veterinary Office, Berne-Liebefeld (as from 1.6.2010)
Dr. Markus Schmutz, Novartis Pharma AG, Basle (as from 30.3.2011)
Silvia Matile-Steiner, lawyer, F. Hoffmann-La Roche Ltd., Basle
Ursula Moser, B.Sc., Federal Veterinary Office, Berne-Liebefeld (until 1.6.2010)
Prof. Hans Wyss, Director of the Federal Veterinary Office, Berne-Liebefeld
The Evaluation Committee
Prof. Peter Maier, University of Zurich (Chairman)
Dr. Franziska Boess, F. Hoffmann-La Roche Ltd, Basle
Prof. Kurt Bürki, Institute of Laboratory Animal Science, University of Zurich (until 31.12.2010)
Prof. Clemens A. Dahinden, Institute of Immunology and Allergology, University Hospital, Berne
Prof. Marianne Geiser Kamber, Institute of Anatomy, University of Berne
Prof. Andrew Hemphill, Institute of Parasitology, University of Berne
Dr. Ingrid Kohler, Federal Veterinary Office, Berne-Liebefeld (as from 1.6.2010)
Dr. Kurt Lingenhöhl, Novartis Pharma Ltd, Basle
Prof. Thomas Lutz, Institute of Veterinary Physiology, University of Zurich
Ursula Moser, B.Sc., Federal Veterinary Office, Berne-Liebefeld (until 1.6.2010)
Dr. Martin Reist, Veterinary Public Health Institute, University of Berne
Dr. Stefanie Schindler, Animalfree Research Foundation, Zurich
Scientific adviser
Prof. Peter Maier, University of Zurich
Administrator
Ernst P. Diener, lawyer, Münsingen
Auditors
Waber Treuhand GmbH, Einigen
Supervisory body
Federal Department of Home Affairs
Articles and statutes of the Foundation
- Deed of foundation dated 13th February, 1987
- Regulations dated 15 May, 1987/30 March, 2011
- Guidelines for awarding research grants dated 15 May, 1987 (latest modification 30 March 2011)
4. Personnel
Ursula Moser, who has represented the Federal Veterinary Office on the Administrative Board and the Evaluation Committee, resigned for professional reasons. Her hard work for both bodies has been much appreciated. Her seats on the Board and the Committee has been taken by Dr. Ingrid Kohler, who also works at the Federal Veterinary Office, for the remaining period of office of 2007/2010. National Councillor Chantal Galladé, Winterthur, and Prof. Paul Herrling, Novartis Pharma AG, Basle, left the Administrative Board, and Prof. Kurt Bürki, Institute of Laboratory Animal Science, University of Zurich, left the Evaluation Committee having completed their periods of office (2007-2010).
5. Activities during 2010
In its twenty-fourth year of existence the Administrative Board met twice, namely in June and December, for a half-day meeting. Apart from the statutory business concerning the end of the business year 2009, the Board addressed the following issues.
Research funds for 2010 were allotted to 14 projects already underway. In addition, 7 new projects were approved, while 18 applications were rejected. The Board also took note of the final assessment by the Evaluation Committee of 3 projects which had been completed in the previous year. An internal working group was set up to review the scope of the Foundation’s activities. In this connection, the Administrative Board is trying to network with other institutions and organisations in the future, in order to achieve greater awareness of the 3R principles. Initial discussions were held with the Swiss National Science Foundation (SNF), the Swiss Academy of Medical Sciences (SAMW) and the Swiss Academy of Natural Sciences (SCNAT), although no concrete agreements have yet been reached.
At its meeting in June, the Board focused on the financial statements for 2009 and the approval of new projects, as well as on those projects that had been completed. Furthermore, it was decided to contact institutions with which the Foundation is keen to network.
At the December Board meeting, the focus was on approving new projects as well as discussing financial issues. Owing to the fact that the necessary funds are not forthcoming, it was not possible to approve all the applications recommended by the Evaluation Committee. A further topic of discussion was the modification of the deed of foundation and the revision of the regulations with the aim of adapting both texts to current circumstances and requirements, as well as making the appeal procedure more practical. The revised texts are to be voted on at the next meeting of the Administrative Board. The Board then went on to discuss the Foundation’s activities in 2010 and efforts to network the Foundation with members of the Evaluation Committee. The meeting was rounded off with a dinner.
The Secretary, now renamed the Administrator, is responsible for the day-to-day running of the Foundation; he deals with all matters that cannot be passed on to anyone else. In particular, he prepares all the necessary information for the Administrative Board to take their decisions, as well as dealing with correspondence with applicants and project managers. He also deals with payments, book-keeping, closing the books at the end of the financial year and the budget. In addition, he prepares the text of the Annual Report as well as texts for the Foundation’s website.
Under the chairmanship of the Scientific Adviser, the Evaluation Committee held two meetings during the year, where in particular they examined 25 new applications for funding and evaluated the 3 completed projects. The voluntary work of the members of the Evaluation Committee in this connection is much appreciated.
The Scientific Adviser's tasks tasks included publishing the 3R-Info-Bulletins (in the form of brochures and on the Foundation’s website at www.forschung3r.ch), writing the brief scientific reports in English which present the projects receiving funding on the Foundation’s website and regularly updating these reports. He was also kept busy – as always – advising applicants and project managers, obtaining intermediate reports, evaluating project outlines, dealing with enquiries and explaining why projects had been rejected. As a co-organiser of the EU START-UP project he helped to prepare the final report. Finally, he represented the Foundation at several scientific meetings in Switzerland and abroad, namely at the Annual General Meeting of the European Consensus Platform for 3R Alternatives to Animal Experimentation in Milan and as a member of the Mirror Group of the EPAA Initiative in Brussels ((for further information visit www.ecopa.eu and ec.europa.eu/enterprise/epaa/index_en.htm).
6. Overview of the number of applications and approvals
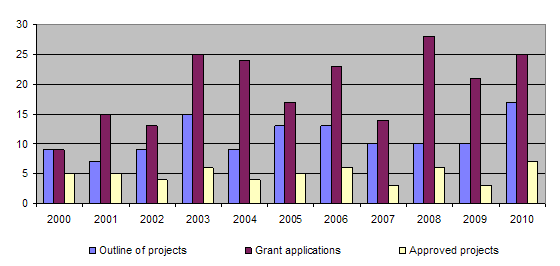
The bar-chart shows that more than 10 outline of projects are submitted each year and over 20 applications are submitted. The proportion of projects approved has varied little over the past few years, with funding being granted for around 5 projects each year. The long-term approval rate for applications is around 30%. Basically, this figure reflects the careful consideration given to each application from the point of view of its relevance to the 3R principles. Consequently, it often happens that projects that are well structured and of considerable scientific interest are not approved for funding because their relevance to the 3R principles is not sufficiently great. In 2010 more projects were recommended for approval by the Evaluation Committee than could in practice be funded. The number of projects approved was therefore limited by the level of available funding.
During the year 3 projects were completed (93/04, 97/05, 105/06). Together with those projects completed earlier, this brings the total of finished projects to 100 out of 124.
7. List of new projects approved in 2010
| 124/10 | Dr. Martin Clauss Orthopaedics Department, Cantonal Hospital Liestal Comparative in vitro and in vivo testing on biofilm formation on the surface of bone grafts |
| 123/10 | Dr. Hans Rufli ecotoxsolutions, Basle Use of “moribund“ stage in the fish acute toxicity test according to OECD guideline 203 and its effect on LC50 values |
| 122/10 | Dr. Helene Rohrbach Department of Clinical Veterinary Medicine, University of Berne Improved perioperative analgesia and reduced stress during recovery for the experimental animal: ultrasound-guided sciatic and femoral nerve block in sheep and quantitative assessment of block quality |
| 121/09 | Prof. Roman Chrast and Prof. Josef Kapfhammer Department of Medical Genetics, University of Lausanne, and Anatomical Institute, University of Basle A new in vitro model to study therapeutic approaches to improve spinal cord regeneration and repair after injury |
| 120/10 | Prof. Denis Jabaudon Department of Basic Neurosciences, University of Geneva Development of non-invasive strategies to study spinal cord disease, injury and repair |
| 119/10 | Dr. Sara Gonzalez Andino Department of Clinical Neurosciences, University of Geneva Non-invasive electrical monitoring of the population spiking activity in the central nervous system |
| 118/10 | Dr. Dalu Mancama CSIR, Biosciences Division, Pretoria, South Africa Engineering of an in vitro hepatocyte tissue system for malaria liver infection research |
A complete list of projects with summaries of each can be found on the Foundation’s website (www.forschung3r.ch/en/projects/index.html).
The brief scientific project reports in English, which are updated once a year, indicate that almost all projects have progressed well. These reports published on the internet are much appreciated by those involved in the research projects as a platform for presenting their work. From the opposite point of view, this system also enables other researchers all over the world to discover new 3R methods without delay.
8. 3R-Info-Bulletin
In 2010 three more new 3R-Info-Bulletins (ISSN 1421-6590) were published in English and distributed to some 1,000 interested parties. The information bulletins are also published on the Foundation’s website (www.forschung3r.ch/en/publications/index.html), as well as in pdf format.
The latest 3R-Info bulletins are:
9. Financial business
A total of some Fr. 730,615 was paid out for research in 2010 (Fr. 728,615.96 for grants to research projects and Fr. 2,000 for participation in conferences). Expenditure on current projects was some Fr. 49,000 over budget (Fr. 679,768.52). On the one hand, Fr. 82,000 budgeted was not spent, but on the other an amount of Fr. 131,000 was already used for newly approved projects. Only Fr. 2,000 out of a budgeted amount of Fr. 6,000 was spent on attendance at conferences.
Operational expenditure for 2010 amounted to Fr. 204,641.05 (project monitoring and information Fr. 102,825.70, administrative costs including office infrastructure Fr. 101,815.35). Total expenditure was therefore around Fr. 14,500 below the budget of Fr. 219,200. This was principally due to a drop in the remuneration of the Administrator and the Scientific Adviser, which is dependent on the amount of work done (- Fr. 8,000) as well as the contribution for method publications (- Fr. 6,000). Administrative costs were some Fr. 9,000 below budget (Fr. 111,200). Total expenditure therefore amounted to Fr. 935,257.01.
On the income side, the equal financial commitment of the federal authorities and Interpharma represented the basic funding for the Foundation’s activities. In 2010 the federal authorities and Interpharma each granted the Foundation Fr. 365,000. As a result of low interest rates, interest on capital was only Fr. 877.75.
Total income was therefore around Fr. 730,973 while total expenditure amounted to Fr. 935,257, giving an excess of expenditure over income of around Fr. 204,283. The unused contributions item fell from approximately Fr. 472,120 at the end of 2009 to Fr. 267,836 at the end of 2010.
At the end of 2010 the total earmarked for projects approved by the Board but not yet paid out amounted to Fr. 1,026,552.65. This future liability is covered by Interpharma’s promise of funding (V). The Foundation’s credit with this institution amounted to Fr. 1,562,000 at the end of 2010.
The budget for 2011 includes around Fr. 648,554 for current projects and a maximum amount of Fr. 500,000 for new projects.
10. Overview of grants awarded between 1987 and 2010
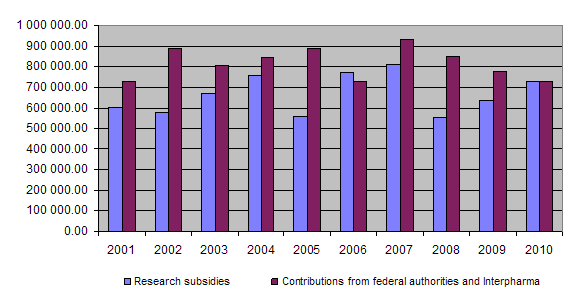
At the end of 2010 a total of Fr. 17,157,960.25 had been granted for projects and other subsidies, of which Fr. 16,131,407.60 had been paid out so far. Together the federal authorities and Interpharma have contributed Fr. 19,176,000 to the Foundation since 1987.
Should the trend seen over the last three years persist, namely a rise in the amount paid out in subsidies and the number of applications worthy of support vis-à-vis a drop in the contributions received from the Confederation and Interpharma, as can be seen from the chart of developments since 2001, it is to be feared that in the future it will not be possible to fund a greater number of 3R-relevant projects.
10-year overview
11. Financial statements
| Profit and loss account 2010 | Expenditure | Income | |
|---|---|---|---|
| Income | |||
| Federal contribution | 365 000.00 | ||
| Contribution from Interpharma | 365 000.00 | ||
| Total contributions | 730 000.00 | ||
| Interest on bank account | 877.75 | ||
| Other income | 96.13 | ||
| Total income | 730 973.88 | ||
Expenditure | |||
| Research grants | 730 615.96 | ||
| Project supervision and information | 102 825.70 | ||
| Administrative expenses | 101 815.35 | ||
| Total expenditure | 935 257.01 | ||
| Excess expenditure over income | - 204 283.13 | ||
| 730 973.88 | |||
| Balance as per 31st December 2010 | Assets | Liabilities | |
| Liquid Assets | |||
| Bank | 422 513.41 | ||
| Accounts payable | 307.20 | ||
| Accounting apportionment assets | 2 639.10 | ||
Liabilities | |||
| Accounting apportionment liabilities | 156 623.05 | ||
| Unused project funds | |||
| Carried forward 1. 1. 2010 | 472 119.79 | ||
| expend. over income | -204 283.13 | 267 836.66 | |
| Capital of the Foundation | 1 000.00 | ||
| 425 459.71 | 556 061.84 | ||
Contingent liabilities | |||
| Approved research grants not yet paid out | Sfr. 1 026 552.65. | ||
Münsingen, 24 February 2011
3R RESEARCH FOUNDATION
Chairwoman: signed C. Egerszegi
Secretary: signed E. Diener
12. Auditors' report to the Administrative Board
Waber Treuhand GmbH in Einigen audited the financial statements for the year according to standards of limited auditing and did not find any indication that the accounts and statements do not correspond to current legislation or the principles and regulations of the Foundation.
| Last modified 2014/06/04 |