
3R-INFO-BULLETIN 40
| | June 2009Authors This new model was developed and characterised by an international team. Dr Anna Bogdanova (Russia) and Dr Johannes Vogel (Germany) are group leaders at the Institute of Veterinary Physiology of the Vetsuisse Faculty and members of the Zurich Centre for Integrative Human Physiology (ZIHP) at the University of Zurich. A PhD student Ms Milena Segato Komniski (Brazil) joined the project for two years. This new model was developed and characterised by an international team. Dr Anna Bogdanova (Russia) and Dr Johannes Vogel (Germany) are group leaders at the Institute of Veterinary Physiology of the Vetsuisse Faculty and members of the Zurich Centre for Integrative Human Physiology (ZIHP) at the University of Zurich. A PhD student Ms Milena Segato Komniski (Brazil) joined the project for two years.
Address: Anna Yu. Bogdanova
annab@access.uzh.ch Johannes Vogel
jvogel@vetphys.uzh.ch Milena Segato Komniski
komniski@vetphys.uzh.ch Institute of Veterinary Physiology
University of Zürich
Winterthurerstrasse 260
CH-8057 Zurich, Switzerland www.vetphys.uzh.ch
Editor
Peter Maier, Scientific Adviser of the 3R Research Foundation |
Refined ex-vivo rodent heart model reduces in vivo experimentation
The present project funded by the 3R Research Foundation (nos. 102-06) focused on validation of a new multifunctional experimental model for the investigation of the responses of healthy and diseased hearts[*] to acute stress or medication. Based on the development of mini-oxygenators the ex-vivo isolated blood-perfused rodent hearts can be used for studies of the myocardial responses to reduction in oxygen supply (hypoxia) and blood flow (ischemia), hyper/hypothermia and in drug testing. It thus reduces the number of animals used for such investigations or can completely replace in-vivo studies.
Blood perfusion is mandatory
To use blood in the Langendorff settings one has to substantially reduce the volume of the circulation circuit. The perfusion volume of a standard Langendorff system is about 100 ml and the priming volume of the smallest commercially available oxygenator is 50 ml. Simple calculations indicate that about 10-12 rats or 60-70 mice have to be sacrificed to obtain the amount of blood required to perfuse a single heart. Washed erythrocytes of a different species (bovine or goat) are sometimes used in combination with a rodent heart. This creates several problems. Artefacts are introduced due to the lack of plasma and white cells in the system. Blood from other species requires extensive washing of the red cells to remove antibodies and other proteins that might induce immune response. These multiple washing steps traumatise red cells and facilitate hemolysis. Hemolysis in turn contributes to the activation and damage of the coronary endothelium and thrombosis. Thus, perfusion with red cells from the same species is the only ultimate solution.
Development of a mini-oxygenator
Substantial reduction of the volume of a circulation circuit is required (from 100 to 5-8 and 0.7-0.5 ml respectively), in order to use blood of the animal to perfuse its own heart. It became possible as two types of hollow fibre mini-oxygenators were developed by J. Vogel for rat and mouse systems respectively. Schematic representation of a perfusion circuit with a mini-oxygenator is shown in Fig 1a and the set-up in the laboratory for rat (Fig1b) and mice (Fig 1c). This setup made it possible to replace the perfusion buffer with blood of the same rodent (rat or mouse) from which the heart was harvested.
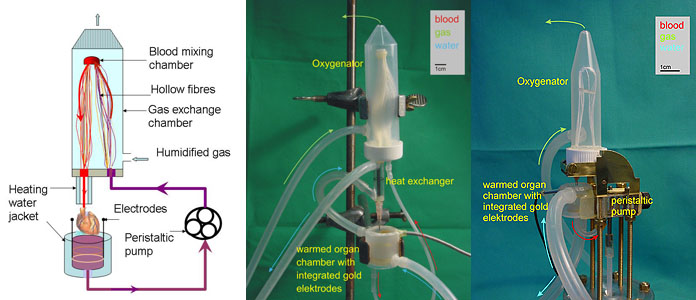
Fig. 1:
1a) Mini-oxygenator scheme
1b) Set-up of the mini-oxygenator for rats
1c) Set-up of the mini-oxygenator for mice
The new miniaturised oxygenators and a novel peristaltic pump in the case of a mouse system reduces the volume of perfusion circuit to 5 ml (for rats) and 0.5 ml (for mouse system), allowing to use one animal for harvesting both blood and heart for a single perfusion experiment. The system allows precise control over the speed of perfusion, blood temperature and blood gas composition. All advantages of the Langendorff perfusion system, including online monitoring of the basic heart parameters during perfusion (electrocardiogram, heart rate and left ventricular pressure), are retained. When perfused at 3 ml blood/min, 37°C, the rat heart beats spontaneously at a normal physiological rate of 277±52 beats per min (mean ±SD, n=14) for at least 90 min without external pacing. Comparison of the selected parameters for the ex-vivo blood-perfused rat heart with the in-vivo values shown in Tables 1 and 2 indicates that our model in many ways perfectly resembles the in-vivo conditions. Higher ATP levels and mild oxidative stress are caused by the lack of load and efficient oxygenation of the isolated organ.
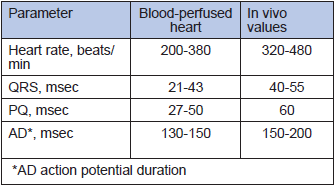
Table 1
Heart rate and electrocardiography in the isolated blood-perfused heart
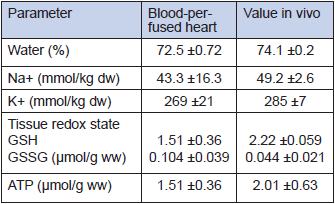
Table 2
Biochemical parameters of the heart tissue in vivo and ex vivo
In vivo-like hypoxic response
In a pilot study we assessed the autonomous response of the heart to hypoxic challenge. These experiments provided further insights into the molecular mechanisms of myocardial responses to hypoxia, including myocardial hibernation and hypoxic preconditioning. Hypoxic stimulus has two faces. Depending on severity and duration, hypoxia may activate defence mechanisms and promote survival (preconditioning) or become a cause of myocardial tissue injury and death (ischemia, myocardial hibernation, and infarction). A blood-perfused rat heart is a perfect model to study autonomous responses of the heart to hypoxia. Several projects currently running in our group focus on various aspects of hypoxic responses such as age-dependent alteration of hypoxia-tolerance, regional changes in metabolism and oxygen sensitivity of the Na,K ATPase in the heart. Shown in Fig 2a is a representative heart rate recording from the senescent heart exposed to hypoxic stress. In contrast to those harvested from young animals, aged heart responds to reduction in blood oxygenation (decrease in blood pO2 from 15 to about 5 kPa) with an instantaneous reduction in heart rate (bradycardia) and increase in action potential duration as follows from the ECG recordings (Fig 2b). If oxygen supply of the aged heart is not restored, bradycardia is followed by loss of contractile function within the next 20-30 min. We currently use this model to select possible pharmacological targets with the final goal to render the old heart more hypoxia-tolerant.
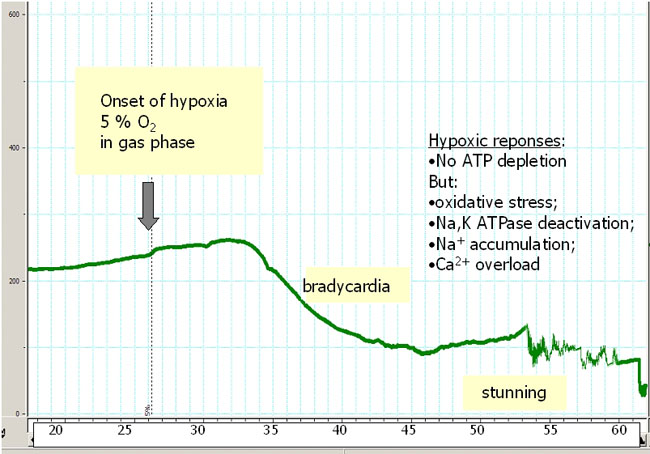
Fig. 2a
Hypoxic responses in the aged heart: Heart rate recording during hypoxia. Not ATP depletion but oxidative stress, Na,K ATPase deactivation, Na+ accumulation and Ca2+ overload cause the hypoxic heart damage.
x-axis: time in minutes; y-axis: heart-beats / min
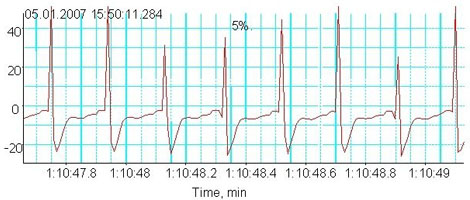
Fig. 2b
Hypoxic responses in the aged heart: Electrocardiogram during hypoxia
Advantages and limitations
By using the mini-oxygenators in the isolated blood-perfused rodent heart model, the number of animals used for our investigations can be reduced by about 80% and even more important reduces the animals’ discomfort level from grade 2-3 to 0-1.
Blood trauma occurring when blood cells pass through the peristaltic pump is a major limiting factor in the presented model. Similar problems arise when using heart-lung machine in clinics but there more interventions can be applied (filtering, conserved blood addition) as perfusion volume makes up several litres. In our system gradual hemolysis and the accumulation of free hemoglobin and K+ in the plasma limit perfusion time to about two hours. However, most of the responses to hypoxia, including recovery phenomena, can be investigated within this time interval. Long-term perfusions would require periodic replacement of the blood in the perfusion circuit.
Ongoing research
Our project is a pilot study designed to characterise a new experimental model. The first findings obtained for the blood-perfused rat system are very promising and this model may be routinely used in future with minor modifications still required to improve the stability and increase the maximal perfusion time. A detailed description of the validation of the isolated autologous blood-perfused rat and mouse heart is currently is submitted to the American Journal of Physiology (5)
PDF version of this Bulletin No. 40
References:
- Abbott CP, Dewitt CW, and Creech O, Jr. The Transplanted Rat Heart: Histologic and Electrocardiographic Changes. Transplantation 3: 432-445, 1965.
- Langendorff O. Untersuchungen am überlebenden Säugetierherzen. Pflugers Arch 61: 291-332, 1898.
- Ono K and Lindsey ES. Improved technique of heart transplantation in rats. The J Thorac Cardiovasc Surg 57: 225-229, 1969.
- Sutherland FJ and Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res 41: 613-627, 2000.
- Segato Komniski M, Makhro A, Gassmann M, Bogdanova A, Vogel J. Isolated autologous blood-perfused heart: validation for rats and mice. submitted to the Am J Physiol 2009.
We gratefully acknowledge Mrs Asya Makhro for the photos and figures.
| [*] | Investigation of cardiovascular dysfunctionsVarious aspects of cardiovascular dysfunctions can be investigated in rodent animal models. Global ischemia-reperfusion injury occurring in patients undergoing cardiac arrest during open heart surgical interventions can be modelled in vivo using a heterotopic rat heart transplantation developed by Abbott in 1965 (1) and modified by Ono and Lindsey in 1969 (3). It includes cardiac arrest, harvesting of the heart of a donor animal and transplantation of it into the abdomen of a recipient animal, where the transplanted heart’s aorta is connected to the recipient’s aorta and its pulmonary artery to the inferior vena cava of the recipient. Global ischemic hypoxia followed by reoxygenation in this model reliably reflects conditions in the cause of ischemia-reperfusion damage observed in patients during on-pump heart surgery. However, it requires two animals per experiment and advanced surgical skills (maximal success rate 75-80%) and is rated as severity grade 2-3 intervention for the recipient animals. Among further disadvantages of this model is its extreme complexity and inability to control precisely blood oxygenation (it depends on the depth of anaesthesia), temperature and pharmacokinetics of the therapeutic agents used. In an attempt to dissect the role of hypoxia-reoxygenation in the development of cold global ischemia-reperfusion injury, the method of choice would be to use an isolated organ model. The most common ex-vivo approach, the so called Langendorff isolated rodent heart preparation, was introduced by Oscar Langendorff more than a century ago (2). The isolated heart is perfused via the aorta with Krebs-Henseleit buffer solution. It is ideal as it allows the examination of cardiac contractile strength (inotropic effects), heart rate (chronotropic effects) and vascular effects without experimental ‘noise’ introduced by the nervous system and hormones as immanent of intact animal models. This model, however, may be the source of artefacts and cannot be used to study hypoxic responses as the oxygen content of the blood-free buffers is 100-fold lower than that of blood (4). The aim of the present project is to develop an ex-vivo model suitable for the investigations of the hyperoxic responses of the rodent heart.
es muss hypoxic heissen nicht hyperoxic. |
 This new model was developed and characterised by an international team. Dr Anna Bogdanova (Russia) and Dr Johannes Vogel (Germany) are group leaders at the Institute of Veterinary Physiology of the Vetsuisse Faculty and members of the Zurich Centre for Integrative Human Physiology (ZIHP) at the University of Zurich. A PhD student Ms Milena Segato Komniski (Brazil) joined the project for two years.
This new model was developed and characterised by an international team. Dr Anna Bogdanova (Russia) and Dr Johannes Vogel (Germany) are group leaders at the Institute of Veterinary Physiology of the Vetsuisse Faculty and members of the Zurich Centre for Integrative Human Physiology (ZIHP) at the University of Zurich. A PhD student Ms Milena Segato Komniski (Brazil) joined the project for two years.




